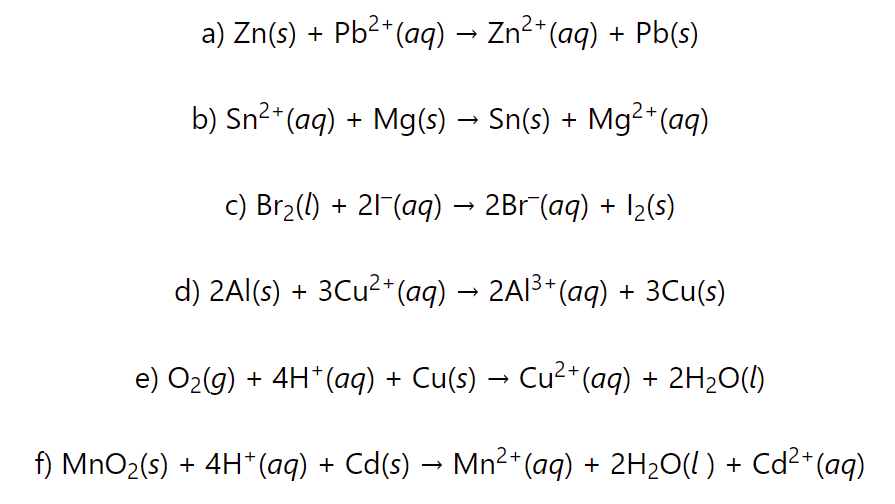Standard Cell Potential Equilibrium Constant . We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a. Free energy and cell potential. example problem for calculating the equilibrium constant k using the standard cell potential. The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and k can be explained by the figure given below: the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. khan academy organic chemistry. Chemistry archive > unit 9. an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. cell potential, free energy, and equilibrium constant.
from general.chemistrysteps.com
Chemistry archive > unit 9. the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. example problem for calculating the equilibrium constant k using the standard cell potential. khan academy organic chemistry. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a. figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. Free energy and cell potential. an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. cell potential, free energy, and equilibrium constant. The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and k can be explained by the figure given below:
Calculate the cell potential, ΔG°, and equilibrium constant K for each reaction Chemistry Steps
Standard Cell Potential Equilibrium Constant We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a. The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and k can be explained by the figure given below: an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. Chemistry archive > unit 9. Free energy and cell potential. example problem for calculating the equilibrium constant k using the standard cell potential. khan academy organic chemistry. figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. cell potential, free energy, and equilibrium constant. the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a.
From www.chegg.com
Solved Cell Potential and Equilibrium Standard reduction Standard Cell Potential Equilibrium Constant Chemistry archive > unit 9. an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. cell potential, free energy, and equilibrium constant. Free energy and cell potential. figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. . Standard Cell Potential Equilibrium Constant.
From www.youtube.com
Electrochemistry 5 Nernst Equation Equilibrium Constant Gibb’s Free Energy Cell Standard Cell Potential Equilibrium Constant an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and k can be explained by the figure given below: cell potential, free energy, and equilibrium constant. . Standard Cell Potential Equilibrium Constant.
From www.numerade.com
Cell potential free energy and equilibrium constant overview Numerade Standard Cell Potential Equilibrium Constant The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and k can be explained by the figure given below: Chemistry archive > unit 9. khan academy organic chemistry. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a. an equilibrium constant of one and cell potential and. Standard Cell Potential Equilibrium Constant.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID321506 Standard Cell Potential Equilibrium Constant figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. example problem for calculating the equilibrium constant k using the standard cell potential. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a.. Standard Cell Potential Equilibrium Constant.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6822021 Standard Cell Potential Equilibrium Constant Chemistry archive > unit 9. an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties. Standard Cell Potential Equilibrium Constant.
From www.slideserve.com
PPT 12.8 Standard Potentials and Equilibrium Constants PowerPoint Presentation ID6754015 Standard Cell Potential Equilibrium Constant Free energy and cell potential. the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. Chemistry archive > unit 9. The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and k can be. Standard Cell Potential Equilibrium Constant.
From www.youtube.com
Equilibrium constant and standard cell potential YouTube Standard Cell Potential Equilibrium Constant an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. Chemistry archive > unit 9. Free energy and cell potential. We have seen, in this chapter, that a. Standard Cell Potential Equilibrium Constant.
From www.youtube.com
The Relationship Between the Standard Cell Potential and the Equilibrium Constant YouTube Standard Cell Potential Equilibrium Constant Chemistry archive > unit 9. figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. Free energy and cell potential. an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. khan academy organic chemistry. The relationship between the. Standard Cell Potential Equilibrium Constant.
From general.chemistrysteps.com
Calculate the cell potential, ΔG°, and equilibrium constant K for each reaction Chemistry Steps Standard Cell Potential Equilibrium Constant Free energy and cell potential. figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. example problem for calculating the equilibrium constant k using the standard cell potential. khan academy organic chemistry. Chemistry archive > unit 9. the connection between cell potential, gibbs energy and constant equilibrium are directly related in the.. Standard Cell Potential Equilibrium Constant.
From www.youtube.com
Standard cell potential and the equilibrium constant Chemistry Khan Academy YouTube Standard Cell Potential Equilibrium Constant the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a. khan academy organic chemistry. example problem for calculating the equilibrium constant k using the standard cell potential. figure \(\pageindex{1}\) summarizes the relationships that we have developed. Standard Cell Potential Equilibrium Constant.
From www.youtube.com
2052 Gibbs Free Energy, Cell Potential, and Equilibrium Constants YouTube Standard Cell Potential Equilibrium Constant Chemistry archive > unit 9. an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. khan academy organic chemistry. figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. cell potential, free energy, and equilibrium constant. The. Standard Cell Potential Equilibrium Constant.
From www.slideserve.com
PPT Chemistry 232 PowerPoint Presentation, free download ID2317752 Standard Cell Potential Equilibrium Constant an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. khan academy organic chemistry. Free energy and cell potential. Chemistry archive > unit 9. cell potential,. Standard Cell Potential Equilibrium Constant.
From www.youtube.com
Free Energy, Equilibrium Constant, and Cell Potential Relationships Explained YouTube Standard Cell Potential Equilibrium Constant an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. Chemistry archive > unit 9. Free energy and cell potential. The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and k can be explained by the figure given. Standard Cell Potential Equilibrium Constant.
From www.youtube.com
Standard cell potential and the equilibrium constant Physical Processes MCAT Khan Academy Standard Cell Potential Equilibrium Constant cell potential, free energy, and equilibrium constant. Chemistry archive > unit 9. an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. example problem for calculating the equilibrium constant k using the standard cell potential. khan academy organic chemistry. The relationship. Standard Cell Potential Equilibrium Constant.
From www.youtube.com
Cell Potential, Free Energy, and Equilibrium YouTube Standard Cell Potential Equilibrium Constant figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a. an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. Free energy and cell. Standard Cell Potential Equilibrium Constant.
From www.studypool.com
SOLUTION AP Chemistry Cell potential & Equilibrium Constant Practice Problems Studypool Standard Cell Potential Equilibrium Constant cell potential, free energy, and equilibrium constant. an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. Free energy and cell potential. example problem for calculating the equilibrium constant k using the standard cell potential. We have seen, in this chapter, that. Standard Cell Potential Equilibrium Constant.
From www.studypool.com
SOLUTION AP Chemistry Cell potential & Equilibrium Constant Practice Problems Studypool Standard Cell Potential Equilibrium Constant example problem for calculating the equilibrium constant k using the standard cell potential. figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. cell potential, free energy, and equilibrium constant. an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium. Standard Cell Potential Equilibrium Constant.
From www.toppr.com
What will be standard cell potential of galvanic cell with the following reaction? 2Cr(s) + 3Cd Standard Cell Potential Equilibrium Constant an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. cell potential, free energy, and equilibrium constant. example problem for calculating the equilibrium constant k using the standard cell potential. Free energy and cell potential. Chemistry archive > unit 9. We have. Standard Cell Potential Equilibrium Constant.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Standard Cell Potential Equilibrium Constant example problem for calculating the equilibrium constant k using the standard cell potential. figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a. The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and. Standard Cell Potential Equilibrium Constant.
From www.youtube.com
Week 13 10. Calculating an equilibrium constant, K, from cell potential! YouTube Standard Cell Potential Equilibrium Constant khan academy organic chemistry. Free energy and cell potential. example problem for calculating the equilibrium constant k using the standard cell potential. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a. Chemistry archive > unit 9. an equilibrium constant of one and cell potential and free energy values equal to zero. Standard Cell Potential Equilibrium Constant.
From app.jove.com
Gibbs Free Energy, Cell Potential and Equilibrium Constant Concept Chemistry JoVe Standard Cell Potential Equilibrium Constant cell potential, free energy, and equilibrium constant. figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. Free energy and cell potential. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a. an equilibrium constant of one and cell potential and free energy values equal to zero is. Standard Cell Potential Equilibrium Constant.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free download ID5480021 Standard Cell Potential Equilibrium Constant figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. khan academy organic chemistry. Chemistry archive > unit 9. an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. cell potential, free energy, and equilibrium constant. . Standard Cell Potential Equilibrium Constant.
From www.toppr.com
Verify that the given functions (explicit or implicit) is a solution of the corresponding Standard Cell Potential Equilibrium Constant figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. example problem for calculating the equilibrium constant k using the standard cell potential. The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and k can be explained by the figure given below: We have seen, in this chapter,. Standard Cell Potential Equilibrium Constant.
From www.youtube.com
Calculating the equilibrium constant from the standard cell potential Khan Academy YouTube Standard Cell Potential Equilibrium Constant Chemistry archive > unit 9. cell potential, free energy, and equilibrium constant. The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and k can be explained by the figure given below: example problem for calculating the equilibrium constant k using the standard cell potential. We have seen, in this chapter, that a. Standard Cell Potential Equilibrium Constant.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free download ID5480021 Standard Cell Potential Equilibrium Constant the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and k can be explained by the figure given below: Free energy and cell potential. an equilibrium constant of one and cell potential and free energy values equal. Standard Cell Potential Equilibrium Constant.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Standard Cell Potential Equilibrium Constant cell potential, free energy, and equilibrium constant. an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. Free energy and cell potential. The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and k can be explained by. Standard Cell Potential Equilibrium Constant.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps Standard Cell Potential Equilibrium Constant figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. example problem for calculating the equilibrium constant k using the standard cell potential. cell potential, free energy, and equilibrium constant. Free energy and cell potential. khan academy organic chemistry. Chemistry archive > unit 9. the connection between cell potential, gibbs energy. Standard Cell Potential Equilibrium Constant.
From slideplayer.com
Electrochemical Cell. ppt download Standard Cell Potential Equilibrium Constant khan academy organic chemistry. figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. Free energy and cell potential. example problem for calculating the equilibrium constant k using the standard cell potential. cell potential, free energy, and equilibrium constant. the connection between cell potential, gibbs energy and constant equilibrium are directly. Standard Cell Potential Equilibrium Constant.
From www.slideserve.com
PPT Electrochemistry MAE295 PowerPoint Presentation, free download ID713597 Standard Cell Potential Equilibrium Constant the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a. Free energy and cell potential. cell potential, free energy, and equilibrium constant. example problem for calculating the equilibrium constant k using the standard cell potential. an. Standard Cell Potential Equilibrium Constant.
From www.jove.com
Gibbs Free Energy, Cell Potential and Equilibrium Constant Chemistry JoVE Standard Cell Potential Equilibrium Constant The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and k can be explained by the figure given below: Chemistry archive > unit 9. the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. cell potential, free energy, and equilibrium constant. We have seen, in this chapter,. Standard Cell Potential Equilibrium Constant.
From www.youtube.com
Calculating the equilibrium constant from the standard cell potential edited Khan Academy Standard Cell Potential Equilibrium Constant example problem for calculating the equilibrium constant k using the standard cell potential. Free energy and cell potential. the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and k can be explained by the figure given below:. Standard Cell Potential Equilibrium Constant.
From www.chegg.com
Solved 4. (a) Calculate the cell potential, equilibrium Standard Cell Potential Equilibrium Constant The relationship between the cell potential under standard conditions and the thermodynamic constants δ g° and k can be explained by the figure given below: the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. cell potential, free energy, and equilibrium constant. khan academy organic chemistry. Free energy and cell potential. . Standard Cell Potential Equilibrium Constant.
From quizspattering.z21.web.core.windows.net
How To Calculate Standard Potential Standard Cell Potential Equilibrium Constant Free energy and cell potential. example problem for calculating the equilibrium constant k using the standard cell potential. cell potential, free energy, and equilibrium constant. khan academy organic chemistry. an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. Chemistry archive. Standard Cell Potential Equilibrium Constant.
From www.researchgate.net
Effect of temperature on standard equilibrium cell potentials for... Download Scientific Diagram Standard Cell Potential Equilibrium Constant We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a. Chemistry archive > unit 9. cell potential, free energy, and equilibrium constant. an equilibrium constant of one and cell potential and free energy values equal to zero is associated with a reaction under equilibrium at standard conditions. The relationship between the cell potential. Standard Cell Potential Equilibrium Constant.
From slideplayer.com
Chapter 17 Electrochemistry ppt download Standard Cell Potential Equilibrium Constant example problem for calculating the equilibrium constant k using the standard cell potential. figure \(\pageindex{1}\) summarizes the relationships that we have developed based on properties of the. the connection between cell potential, gibbs energy and constant equilibrium are directly related in the. khan academy organic chemistry. cell potential, free energy, and equilibrium constant. Free energy. Standard Cell Potential Equilibrium Constant.