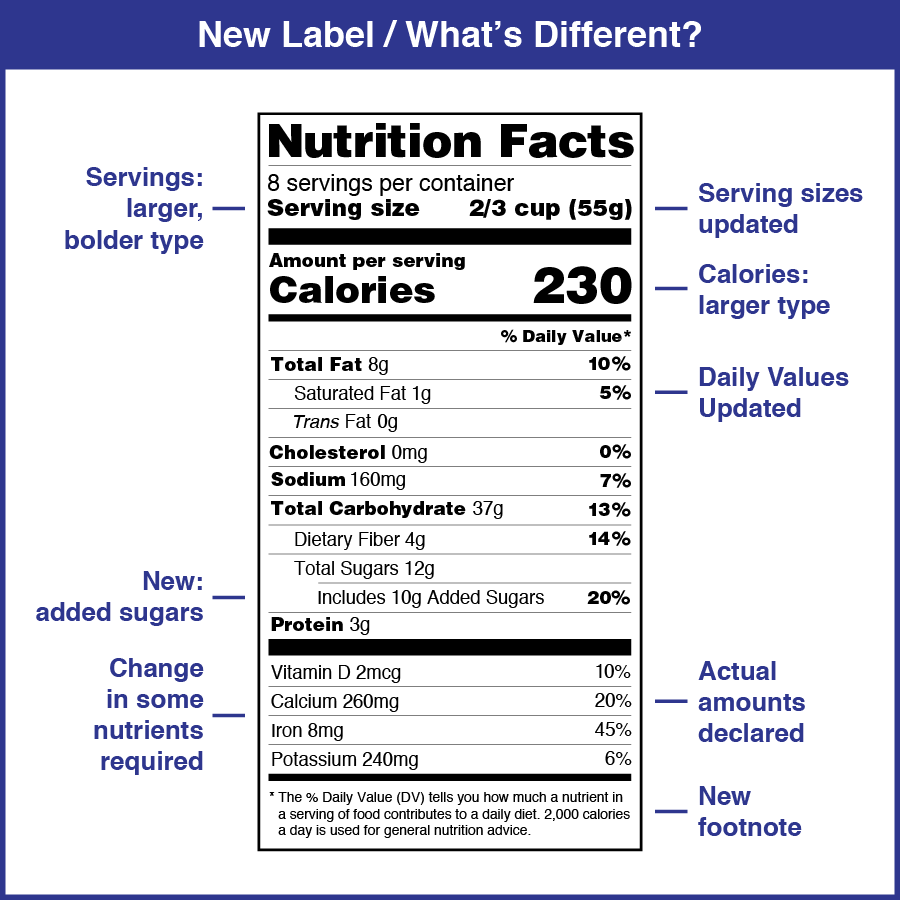
Drug Labelling Guidelines Usfda . Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: (1) the labeling must contain a summary of the. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. Search for labels on dailymed. For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. The labels are also available on the national library of medicine's dailymed web site.
from www.fdalisting.com
Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. The labels are also available on the national library of medicine's dailymed web site. Search for labels on dailymed. For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample. (1) the labeling must contain a summary of the. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements:
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements
Drug Labelling Guidelines Usfda (1) the labeling must contain a summary of the. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample. (1) the labeling must contain a summary of the. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Search for labels on dailymed. The labels are also available on the national library of medicine's dailymed web site. Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy.
From www.fda.gov
The OvertheCounter Medicine Label Take a Look FDA Drug Labelling Guidelines Usfda Search for labels on dailymed. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. The labels are also available on the national library of medicine's dailymed web site. For more information. Drug Labelling Guidelines Usfda.
From foodindustryexecutive.com
FDA Final Guidance Clarifies New Nutrition Label Requirements Food Drug Labelling Guidelines Usfda Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. Search for labels on dailymed. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. (1) the labeling must contain a summary of the. The labels are also available on. Drug Labelling Guidelines Usfda.
From blog.catalpha.com
Understanding FDA Labeling Requirements For Food Products Drug Labelling Guidelines Usfda Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. The labels are also available on the national library of medicine's dailymed web site. For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample. (1) the labeling must contain a summary of the. Fda guidance documents discuss. Drug Labelling Guidelines Usfda.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labelling Guidelines Usfda Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. Search for labels on dailymed. This guidance is. Drug Labelling Guidelines Usfda.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labelling Guidelines Usfda Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. Search for labels on dailymed. (1) the labeling must contain a summary of the. This guidance is intended to assist applicants in complying. Drug Labelling Guidelines Usfda.
From almetaygertrudis.pages.dev
Amp 2024 Usfda Guidelines Aline Beitris Drug Labelling Guidelines Usfda Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (1) the labeling must contain a summary of the. Search for labels on dailymed. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Contain a summary of essential scientific information. Drug Labelling Guidelines Usfda.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labelling Guidelines Usfda For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. Human prescription drug labeling (1) contains a summary of the essential scientific. Drug Labelling Guidelines Usfda.
From exocyqbgg.blob.core.windows.net
Drug Facts Labels Of Us Fda Pdf at Diana Swank blog Drug Labelling Guidelines Usfda Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: The labels are also available on. Drug Labelling Guidelines Usfda.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labelling Guidelines Usfda The labels are also available on the national library of medicine's dailymed web site. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Contain a summary of essential scientific information needed. Drug Labelling Guidelines Usfda.
From www.artworkflowhq.com
Your Goto Handbook of FDA’s Labeling Requirements For Dietary Supplements Drug Labelling Guidelines Usfda For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. Prescription drug labeling described in § 201.100 (d). Drug Labelling Guidelines Usfda.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labelling Guidelines Usfda Search for labels on dailymed. Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Fda. Drug Labelling Guidelines Usfda.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labelling Guidelines Usfda This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. (1) the labeling must contain a summary of the. Search for labels on dailymed. For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample. Contain a summary of essential scientific information needed for safe and effective. Drug Labelling Guidelines Usfda.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Drug Labelling Guidelines Usfda For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: (1) the labeling must contain a summary of the. The labels are. Drug Labelling Guidelines Usfda.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labelling Guidelines Usfda This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample. Search for labels on dailymed. (1) the. Drug Labelling Guidelines Usfda.
From www.fdalisting.com
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements Drug Labelling Guidelines Usfda Search for labels on dailymed. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. (1) the labeling must contain a summary of the. The labels are also available on the national. Drug Labelling Guidelines Usfda.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Drug Labelling Guidelines Usfda Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (1) the labeling must contain a summary of the. Search for labels on dailymed. Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. Prescription drug labeling described in § 201.100. Drug Labelling Guidelines Usfda.
From foodsafetystandard.in
Food Safety Standard Drug Labelling Guidelines Usfda Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Human prescription drug labeling (1) contains a summary. Drug Labelling Guidelines Usfda.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Drug Labelling Guidelines Usfda The labels are also available on the national library of medicine's dailymed web site. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. Prescription drug labeling described in § 201.100 (d). Drug Labelling Guidelines Usfda.
From exocyqbgg.blob.core.windows.net
Drug Facts Labels Of Us Fda Pdf at Diana Swank blog Drug Labelling Guidelines Usfda Search for labels on dailymed. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. (1) the labeling must contain a summary of the. The labels are also available on the national library of medicine's dailymed web site. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and. Drug Labelling Guidelines Usfda.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labelling Guidelines Usfda Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. (1) the labeling must contain a summary of the. Contain a summary of. Drug Labelling Guidelines Usfda.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Drug Labelling Guidelines Usfda (1) the labeling must contain a summary of the. The labels are also available on the national library of medicine's dailymed web site. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug. Drug Labelling Guidelines Usfda.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labelling Guidelines Usfda For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample. Search for labels on dailymed. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: The labels are also available on the national library of medicine's dailymed web site. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote. Drug Labelling Guidelines Usfda.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labelling Guidelines Usfda Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. Search for labels. Drug Labelling Guidelines Usfda.
From exokgfcfa.blob.core.windows.net
Drug Facts Label Of Usfda Assignment Pdf at Belinda Diaz blog Drug Labelling Guidelines Usfda Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: (1) the labeling must contain a summary of the. This guidance is intended to assist applicants in complying with the content and format requirements of labeling. Drug Labelling Guidelines Usfda.
From www.fdalisting.com
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements Drug Labelling Guidelines Usfda Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This guidance is. Drug Labelling Guidelines Usfda.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Drug Labelling Guidelines Usfda Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. The labels are also available on the national library of medicine's dailymed web site. (1) the labeling must contain a. Drug Labelling Guidelines Usfda.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Drug Labelling Guidelines Usfda (1) the labeling must contain a summary of the. Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. The labels are also available on the national library of medicine's dailymed web. Drug Labelling Guidelines Usfda.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Drug Labelling Guidelines Usfda Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample. This guidance is intended to assist applicants in complying. Drug Labelling Guidelines Usfda.
From www.slideserve.com
PPT FDA LABELING PowerPoint Presentation, free download ID3633953 Drug Labelling Guidelines Usfda Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Human prescription drug labeling (1) contains a summary. Drug Labelling Guidelines Usfda.
From www.onlinelabels.com
What You Need to Know About the New FDA Nutrition Fact Label Drug Labelling Guidelines Usfda Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. (1) the labeling must contain a summary of. Drug Labelling Guidelines Usfda.
From www.icoptix.com
Labeling Laws/FDA and EU Guidance IC Optix Drug Labelling Guidelines Usfda Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. The labels are also available on the national library of medicine's dailymed web site. For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and. Drug Labelling Guidelines Usfda.
From www.artworkflowhq.com
Pharmaceutical Labeling 101 FDA Drug Labelling Regulations Guide [2024 Drug Labelling Guidelines Usfda For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This guidance is intended to assist applicants in complying with the content and format requirements of labeling for human prescription. Contain a summary of essential scientific information. Drug Labelling Guidelines Usfda.
From www.pharmaceutical-technology.com
FDA revises biosimilar guidelines for clearer drug labelling Drug Labelling Guidelines Usfda Search for labels on dailymed. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Contain a summary of essential scientific information needed for safe and effective use of the human prescription drug or biological. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking. Drug Labelling Guidelines Usfda.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Drug Labelling Guidelines Usfda (1) the labeling must contain a summary of the. Fda guidance documents discuss the production, labeling, manufacturing of regulated products and denote fda's current thinking and policy. Search for labels on dailymed. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: For more information on labeling, including physician labeling rule (plr) requirements, guidances, presentations, sample.. Drug Labelling Guidelines Usfda.
From animalia-life.club
Fda Drug Labeling Requirements Drug Labelling Guidelines Usfda The labels are also available on the national library of medicine's dailymed web site. Search for labels on dailymed. (1) the labeling must contain a summary of the. Prescription drug labeling described in § 201.100 (d) must meet the following general requirements: Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and. Drug Labelling Guidelines Usfda.