Magnesium Ion Oxidation Number . To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: A net ionic charge can be specified at the end of the. Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. Enter the formula of a chemical compound to find the oxidation number of each element. Assigning oxidation states to the elements in binary ionic compounds is straightforward: The oxidation states of the elements are identical to the charges on the monatomic. The oxidation number tells us how many electrons have been removed in order to form a chemical bond, so two lost electrons means an oxidation. The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a.
from www.slideserve.com
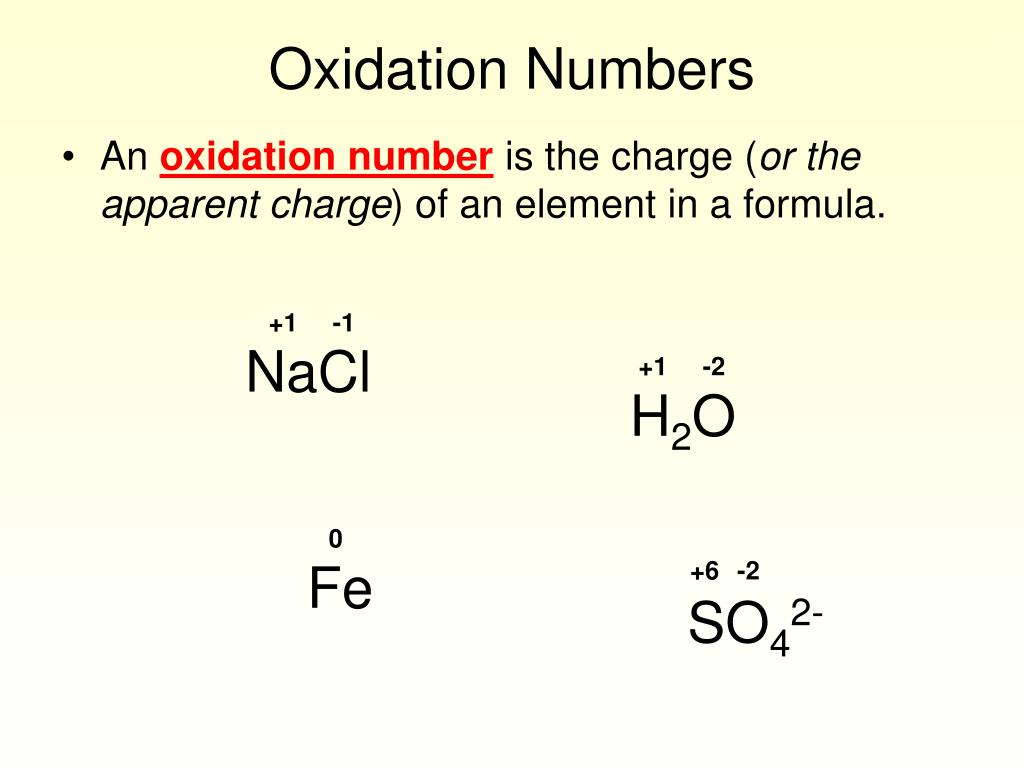
The oxidation states of the elements are identical to the charges on the monatomic. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. Enter the formula of a chemical compound to find the oxidation number of each element. Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to. The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. Assigning oxidation states to the elements in binary ionic compounds is straightforward: A net ionic charge can be specified at the end of the. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: The oxidation number tells us how many electrons have been removed in order to form a chemical bond, so two lost electrons means an oxidation.
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015
Magnesium Ion Oxidation Number Enter the formula of a chemical compound to find the oxidation number of each element. A net ionic charge can be specified at the end of the. Assigning oxidation states to the elements in binary ionic compounds is straightforward: The oxidation states of the elements are identical to the charges on the monatomic. The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to. Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation number tells us how many electrons have been removed in order to form a chemical bond, so two lost electrons means an oxidation.
From rodolfozebrivera.blogspot.com
Oxidation Number of Magnesium RodolfozebRivera Magnesium Ion Oxidation Number Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing. Magnesium Ion Oxidation Number.
From www.numerade.com
SOLVED What is the oxidation number of sulfur in magnesium sulfite, MgSO3? Magnesium Ion Oxidation Number The oxidation number tells us how many electrons have been removed in order to form a chemical bond, so two lost electrons means an oxidation. Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its. Magnesium Ion Oxidation Number.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 Magnesium Ion Oxidation Number Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. The oxidation number tells us how many electrons have been removed in order to form a chemical bond, so two lost electrons means an oxidation. Assigning oxidation states to the elements in binary ionic compounds is straightforward: The oxidation state of an atom. Magnesium Ion Oxidation Number.
From socratic.org
Which one of the elements with following outer electronic Magnesium Ion Oxidation Number The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. Oxidation numbers are used to track how many electrons are lost or gained in a chemical. Magnesium Ion Oxidation Number.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID270027 Magnesium Ion Oxidation Number The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. Assigning oxidation states to the elements in binary ionic compounds is straightforward: To calculate oxidation numbers of elements in the. Magnesium Ion Oxidation Number.
From www.alamy.com
Diagram to show ionic bonding in magnesium oxide Stock Vector Image Magnesium Ion Oxidation Number Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. To calculate oxidation numbers of elements in. Magnesium Ion Oxidation Number.
From nuevaescuelamexicana.org
Dominando los números de oxidación Guía para comprender su importancia Magnesium Ion Oxidation Number Assigning oxidation states to the elements in binary ionic compounds is straightforward: To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to. A net ionic charge can be specified at. Magnesium Ion Oxidation Number.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Ion Oxidation Number To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Assigning oxidation states to the elements in binary ionic compounds is straightforward: The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. Oxidation numbers are used to track how. Magnesium Ion Oxidation Number.
From www.nagwa.com
Question Video Identifying If Magnesium Is Undergoing Oxidization or Magnesium Ion Oxidation Number The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to. Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation. Magnesium Ion Oxidation Number.
From www.pinterest.com
Oxidation of Magnesium,Mg Magnesium, Oxidation, Oxidation state Magnesium Ion Oxidation Number A net ionic charge can be specified at the end of the. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: The oxidation states of the elements are identical to the charges on the monatomic. The oxidation state of an atom is equal to the total number of electrons which have. Magnesium Ion Oxidation Number.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:1.6 4 Ionic Bonds Dot Magnesium Ion Oxidation Number The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to. Assigning oxidation states to the elements in binary ionic compounds is straightforward: Enter the formula of a. Magnesium Ion Oxidation Number.
From articleeducation.x.fc2.com
Explain how magnesium and oxygen atoms react to form magnesium oxide Magnesium Ion Oxidation Number The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. The oxidation number tells us how many electrons have been removed in order to form a chemical bond, so two lost electrons means an oxidation. The oxidation states of the elements are identical to the charges on. Magnesium Ion Oxidation Number.
From rodolfozebrivera.blogspot.com
Oxidation Number of Magnesium RodolfozebRivera Magnesium Ion Oxidation Number Assigning oxidation states to the elements in binary ionic compounds is straightforward: Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to. Enter the formula of a chemical compound to find the oxidation number of each element. To calculate oxidation numbers of elements in the chemical compound, enter. Magnesium Ion Oxidation Number.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Ion Oxidation Number A net ionic charge can be specified at the end of the. The oxidation number tells us how many electrons have been removed in order to form a chemical bond, so two lost electrons means an oxidation. Assigning oxidation states to the elements in binary ionic compounds is straightforward: The oxidation number of a monatomic ion (by itself or as. Magnesium Ion Oxidation Number.
From www.youtube.com
How to find the Oxidation Number for N in Mg(NO3)2 (Magnesium nitrate Magnesium Ion Oxidation Number Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to. The oxidation states of the elements are identical to the charges on the monatomic. Enter the formula of a chemical compound to find the oxidation number of each element. A net ionic charge can be specified at the. Magnesium Ion Oxidation Number.
From www.slideserve.com
PPT Electrochemistry Lesson 3 Oxidation Numbers PowerPoint Magnesium Ion Oxidation Number Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation states of the elements are identical to the charges on the monatomic. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and. Magnesium Ion Oxidation Number.
From sites.google.com
Atomic Structure Protons, Neutrons and Electrons Mrs. Sanborn's Site Magnesium Ion Oxidation Number Assigning oxidation states to the elements in binary ionic compounds is straightforward: A net ionic charge can be specified at the end of the. Enter the formula of a chemical compound to find the oxidation number of each element. Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. The oxidation states of. Magnesium Ion Oxidation Number.
From rodolfozebrivera.blogspot.com
Oxidation Number of Magnesium RodolfozebRivera Magnesium Ion Oxidation Number The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. A net ionic charge can be specified at the end of the. Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to. The oxidation states. Magnesium Ion Oxidation Number.
From ar.inspiredpencil.com
Periodic Table Of Elements With Oxidation Numbers Magnesium Ion Oxidation Number To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to. Magnesium Ion Oxidation Number.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Ion Oxidation Number The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. Enter the formula of a chemical compound to find the oxidation number of each element. Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. To calculate oxidation numbers of elements in. Magnesium Ion Oxidation Number.
From www.tessshebaylo.com
Chemical Equation For Synthesis Of Magnesium Oxide From And Oxygen Magnesium Ion Oxidation Number The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. Assigning oxidation states to the elements in binary ionic compounds is straightforward: Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. Oxidation state shows the total number of electrons. Magnesium Ion Oxidation Number.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor Magnesium Ion Oxidation Number Assigning oxidation states to the elements in binary ionic compounds is straightforward: A net ionic charge can be specified at the end of the. Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to. The oxidation number tells us how many electrons have been removed in order to. Magnesium Ion Oxidation Number.
From www.shemmassianconsulting.com
Oxidation and Reduction Reactions for the MCAT Everything You Need to Magnesium Ion Oxidation Number To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. Assigning oxidation states to the elements in binary ionic compounds is straightforward: The oxidation state of an atom is equal to. Magnesium Ion Oxidation Number.
From www.newtondesk.com
magnesium electron configuration Newton Desk Magnesium Ion Oxidation Number To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to. The oxidation number tells us how many electrons have been removed in order to form a chemical bond, so two. Magnesium Ion Oxidation Number.
From www.slideserve.com
PPT Rules for assigning Oxidation numbers PowerPoint Presentation Magnesium Ion Oxidation Number A net ionic charge can be specified at the end of the. The oxidation states of the elements are identical to the charges on the monatomic. The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. The oxidation state of an atom is equal to the total number of. Magnesium Ion Oxidation Number.
From www.slideserve.com
PPT Hydrogen Gas from the Reaction of Magnesium Metal With Acid Magnesium Ion Oxidation Number The oxidation states of the elements are identical to the charges on the monatomic. A net ionic charge can be specified at the end of the. The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. Assigning oxidation states to the elements in binary ionic compounds is straightforward: To. Magnesium Ion Oxidation Number.
From medium.com
What is Magnesium? Periodic Table Elements Medium Periodic Table Magnesium Ion Oxidation Number The oxidation states of the elements are identical to the charges on the monatomic. The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. A net ionic charge can be specified at. Magnesium Ion Oxidation Number.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Oxidation Number To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: The oxidation number tells us how many electrons have been removed in order to form a chemical bond, so two lost electrons means an oxidation. The oxidation state of an atom is equal to the total number of electrons which have been. Magnesium Ion Oxidation Number.
From www.youtube.com
Oxidation reaction of Magnesium Reaction of Magnesium with Oxygen Magnesium Ion Oxidation Number The oxidation states of the elements are identical to the charges on the monatomic. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Enter the formula of a. Magnesium Ion Oxidation Number.
From www.sliderbase.com
Redox Reactions Presentation Chemistry Magnesium Ion Oxidation Number The oxidation number tells us how many electrons have been removed in order to form a chemical bond, so two lost electrons means an oxidation. Oxidation numbers are used to track how many electrons are lost or gained in a chemical reactions. The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal. Magnesium Ion Oxidation Number.
From arturo-blake.blogspot.com
ArturoBlake Magnesium Ion Oxidation Number The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. Assigning oxidation states to the elements in binary ionic compounds is straightforward: Enter the formula of a chemical compound to find the oxidation number of each element. To calculate oxidation numbers of elements in the chemical compound,. Magnesium Ion Oxidation Number.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Ion Oxidation Number The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. The oxidation number tells us how many electrons have been removed in order to form a chemical bond, so two lost electrons means an oxidation. Oxidation numbers are used to track how many electrons are lost or gained in. Magnesium Ion Oxidation Number.
From www.youtube.com
How to find the Oxidation Number for the Mg2+ ion. (Magnesium ion Magnesium Ion Oxidation Number Oxidation state shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to. The oxidation number tells us how many electrons have been removed in order to form a chemical bond, so two lost electrons means an oxidation. Assigning oxidation states to the elements in binary ionic compounds is straightforward: A. Magnesium Ion Oxidation Number.
From www.youtube.com
Magnesium oxidation number in Mg3N2 ( magnesium nitride ) YouTube Magnesium Ion Oxidation Number Enter the formula of a chemical compound to find the oxidation number of each element. The oxidation number of a monatomic ion (by itself or as part of an ionic compound) is equal to its charge. The oxidation number tells us how many electrons have been removed in order to form a chemical bond, so two lost electrons means an. Magnesium Ion Oxidation Number.
From www.slideserve.com
PPT Valence Electrons and Oxidation Numbers PowerPoint Presentation Magnesium Ion Oxidation Number A net ionic charge can be specified at the end of the. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. The oxidation number tells us how many electrons have been removed in order to form a chemical bond, so two lost electrons means an oxidation.. Magnesium Ion Oxidation Number.