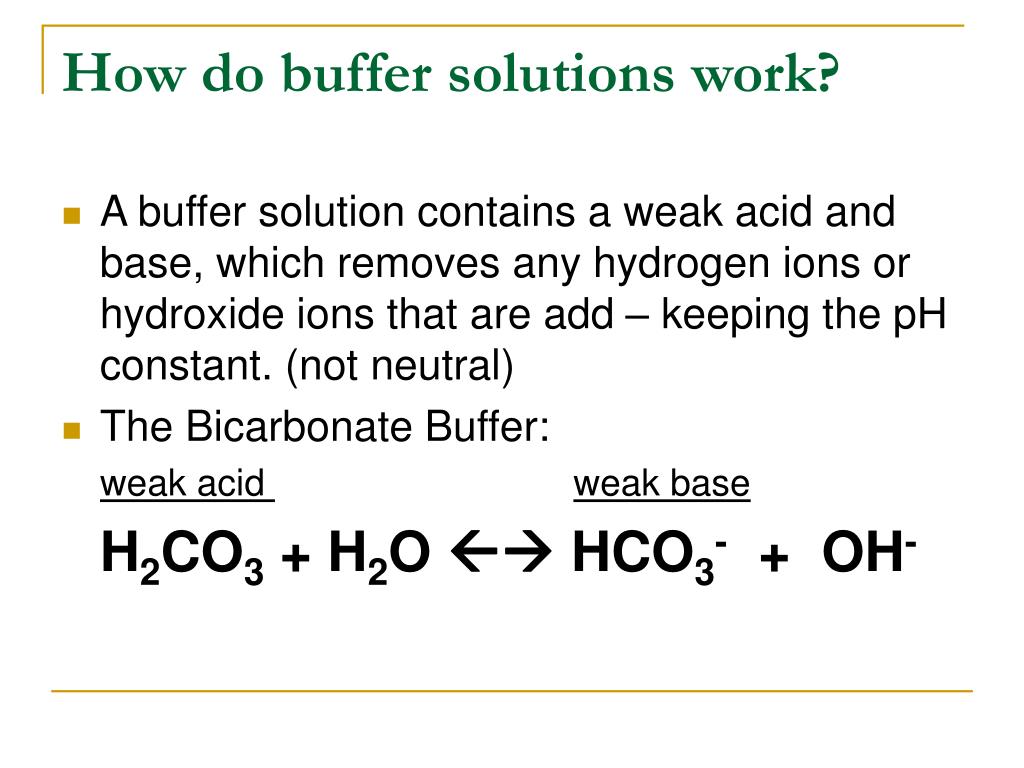
How To Make Buffer More Acidic . A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. How to excel at calculations for acidic buffers using the acid dissociation constant. A common example would be a mixture of ethanoic acid and sodium ethanoate in. It is able to neutralize small amounts of added acid or base, thus. Le chatelier’s principle can be used to predict the effect on the equilibrium position of the solution. How to understand acidic buffers and their function. Learn how buffers protect against ph changes when strong acid or base is added. A buffer solution is an aqueous solution that resists ph change. Learn how to choose an acid/base pair and determine the ratio of their concentrations to make a buffer solution with a given ph. A buffer solution requires a weak acid/base conjugate pair, as opposed to a strong acid and its conjugate,. To understand how buffers work, let’s look first at how the ionization equilibrium of a weak acid is affected by adding either the conjugate base of the acid or a strong acid (a source of \(h^+\)). A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.
from www.slideserve.com
It is able to neutralize small amounts of added acid or base, thus. Learn how to choose an acid/base pair and determine the ratio of their concentrations to make a buffer solution with a given ph. A common example would be a mixture of ethanoic acid and sodium ethanoate in. A buffer solution is an aqueous solution that resists ph change. How to excel at calculations for acidic buffers using the acid dissociation constant. A buffer solution requires a weak acid/base conjugate pair, as opposed to a strong acid and its conjugate,. How to understand acidic buffers and their function. Learn how buffers protect against ph changes when strong acid or base is added. Le chatelier’s principle can be used to predict the effect on the equilibrium position of the solution. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or.
PPT Buffers PowerPoint Presentation, free download ID5687114
How To Make Buffer More Acidic Learn how buffers protect against ph changes when strong acid or base is added. A buffer solution requires a weak acid/base conjugate pair, as opposed to a strong acid and its conjugate,. It is able to neutralize small amounts of added acid or base, thus. Learn how to choose an acid/base pair and determine the ratio of their concentrations to make a buffer solution with a given ph. How to understand acidic buffers and their function. Le chatelier’s principle can be used to predict the effect on the equilibrium position of the solution. To understand how buffers work, let’s look first at how the ionization equilibrium of a weak acid is affected by adding either the conjugate base of the acid or a strong acid (a source of \(h^+\)). Learn how buffers protect against ph changes when strong acid or base is added. A buffer solution is an aqueous solution that resists ph change. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A common example would be a mixture of ethanoic acid and sodium ethanoate in. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. How to excel at calculations for acidic buffers using the acid dissociation constant.
From www.youtube.com
What if Buffer Solution Types of Buffer Solution Acidic Buffer How To Make Buffer More Acidic A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. To understand how buffers work, let’s look first at how the ionization equilibrium of a weak acid is affected by adding either the conjugate base of the acid or a strong acid (a. How To Make Buffer More Acidic.
From www.youtube.com
Buffer Action Acidic and basic buffer Action how buffer resist pH How To Make Buffer More Acidic How to understand acidic buffers and their function. It is able to neutralize small amounts of added acid or base, thus. Le chatelier’s principle can be used to predict the effect on the equilibrium position of the solution. Learn how buffers protect against ph changes when strong acid or base is added. A buffer solution is an aqueous solution that. How To Make Buffer More Acidic.
From www.slideserve.com
PPT Buffers & Buffer Capacity PowerPoint Presentation ID5949383 How To Make Buffer More Acidic How to understand acidic buffers and their function. A buffer solution is an aqueous solution that resists ph change. A common example would be a mixture of ethanoic acid and sodium ethanoate in. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or.. How To Make Buffer More Acidic.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation How To Make Buffer More Acidic A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer solution is an aqueous solution that resists ph change. Le chatelier’s principle can be used to predict the effect on the equilibrium position of the solution. A common example would be. How To Make Buffer More Acidic.
From courses.lumenlearning.com
Buffers Chemistry How To Make Buffer More Acidic To understand how buffers work, let’s look first at how the ionization equilibrium of a weak acid is affected by adding either the conjugate base of the acid or a strong acid (a source of \(h^+\)). A common example would be a mixture of ethanoic acid and sodium ethanoate in. Le chatelier’s principle can be used to predict the effect. How To Make Buffer More Acidic.
From chemistrytalk.org
What is a Buffer Solution? Chemistry ChemTalk How To Make Buffer More Acidic Le chatelier’s principle can be used to predict the effect on the equilibrium position of the solution. Learn how to choose an acid/base pair and determine the ratio of their concentrations to make a buffer solution with a given ph. Learn how buffers protect against ph changes when strong acid or base is added. A buffer solution is an aqueous. How To Make Buffer More Acidic.
From www.aakash.ac.in
Acidic Buffer Solution Definition, Calculation & Applications How To Make Buffer More Acidic A common example would be a mixture of ethanoic acid and sodium ethanoate in. Learn how buffers protect against ph changes when strong acid or base is added. How to understand acidic buffers and their function. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a. How To Make Buffer More Acidic.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 How To Make Buffer More Acidic A buffer solution is an aqueous solution that resists ph change. It is able to neutralize small amounts of added acid or base, thus. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A common example would be a mixture of ethanoic acid and sodium ethanoate in. Le chatelier’s principle. How To Make Buffer More Acidic.
From www.vrogue.co
Ppt Assignment Acidic Basic And Neutral Solutions Pow vrogue.co How To Make Buffer More Acidic Learn how buffers protect against ph changes when strong acid or base is added. How to excel at calculations for acidic buffers using the acid dissociation constant. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Le chatelier’s principle can be used. How To Make Buffer More Acidic.
From www.youtube.com
What is buffer types of buffer Acidic buffer and basic buffer How To Make Buffer More Acidic How to excel at calculations for acidic buffers using the acid dissociation constant. It is able to neutralize small amounts of added acid or base, thus. Learn how buffers protect against ph changes when strong acid or base is added. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Learn. How To Make Buffer More Acidic.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint How To Make Buffer More Acidic A buffer solution requires a weak acid/base conjugate pair, as opposed to a strong acid and its conjugate,. How to understand acidic buffers and their function. Le chatelier’s principle can be used to predict the effect on the equilibrium position of the solution. Learn how buffers protect against ph changes when strong acid or base is added. Learn how to. How To Make Buffer More Acidic.
From www.pinterest.com
Pin on bch How To Make Buffer More Acidic How to excel at calculations for acidic buffers using the acid dissociation constant. How to understand acidic buffers and their function. To understand how buffers work, let’s look first at how the ionization equilibrium of a weak acid is affected by adding either the conjugate base of the acid or a strong acid (a source of \(h^+\)). A buffer solution. How To Make Buffer More Acidic.
From magmastory.blogspot.com
Which Of The Following Represents A Buffer System? magmastory How To Make Buffer More Acidic A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is an aqueous solution that resists ph change. It is able to neutralize small amounts of added acid or base, thus. How to excel at calculations for acidic buffers using the acid dissociation constant. A common example would. How To Make Buffer More Acidic.
From lifeboat.com
Acids, Bases, PH, Buffers How To Make Buffer More Acidic Le chatelier’s principle can be used to predict the effect on the equilibrium position of the solution. To understand how buffers work, let’s look first at how the ionization equilibrium of a weak acid is affected by adding either the conjugate base of the acid or a strong acid (a source of \(h^+\)). Learn how to choose an acid/base pair. How To Make Buffer More Acidic.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5845696 How To Make Buffer More Acidic A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer solution is an aqueous solution that resists ph change. How to understand acidic buffers and their function. A buffer solution requires a weak acid/base conjugate pair, as opposed to a strong. How To Make Buffer More Acidic.
From www.chemistrynotmystery.com
How to Prepare Buffer Solutions? Chemistry!!! Not Mystery How To Make Buffer More Acidic How to understand acidic buffers and their function. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Le chatelier’s principle can be used to predict the effect on the equilibrium position of the solution. To understand how buffers work, let’s look first at how the ionization equilibrium of a weak. How To Make Buffer More Acidic.
From design.udlvirtual.edu.pe
How Does A Circular Buffer Work Design Talk How To Make Buffer More Acidic A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Le chatelier’s principle can be used to predict the effect on the equilibrium position of the solution. A common example would be a mixture of ethanoic acid and sodium ethanoate in. How to. How To Make Buffer More Acidic.
From www.youtube.com
How to prepare acidic buffer solution? YouTube How To Make Buffer More Acidic Learn how to choose an acid/base pair and determine the ratio of their concentrations to make a buffer solution with a given ph. A common example would be a mixture of ethanoic acid and sodium ethanoate in. A buffer solution requires a weak acid/base conjugate pair, as opposed to a strong acid and its conjugate,. A buffer is a solution. How To Make Buffer More Acidic.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded How To Make Buffer More Acidic Le chatelier’s principle can be used to predict the effect on the equilibrium position of the solution. A buffer solution requires a weak acid/base conjugate pair, as opposed to a strong acid and its conjugate,. A buffer solution is an aqueous solution that resists ph change. A common example would be a mixture of ethanoic acid and sodium ethanoate in.. How To Make Buffer More Acidic.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint How To Make Buffer More Acidic A buffer solution requires a weak acid/base conjugate pair, as opposed to a strong acid and its conjugate,. How to understand acidic buffers and their function. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Learn how to choose an acid/base pair. How To Make Buffer More Acidic.
From www.youtube.com
Acidic and Basic buffer Identification and Preparation Tips and Tricks How To Make Buffer More Acidic To understand how buffers work, let’s look first at how the ionization equilibrium of a weak acid is affected by adding either the conjugate base of the acid or a strong acid (a source of \(h^+\)). A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution is an. How To Make Buffer More Acidic.
From www.coursehero.com
[Solved] 1. Explain what is in a buffer. Discuss the function of a How To Make Buffer More Acidic How to excel at calculations for acidic buffers using the acid dissociation constant. To understand how buffers work, let’s look first at how the ionization equilibrium of a weak acid is affected by adding either the conjugate base of the acid or a strong acid (a source of \(h^+\)). Learn how buffers protect against ph changes when strong acid or. How To Make Buffer More Acidic.
From www.chegg.com
Solved Calculating the pH of a Buffer Solution To prepare a How To Make Buffer More Acidic How to excel at calculations for acidic buffers using the acid dissociation constant. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Le chatelier’s principle can be used to predict the effect on the equilibrium position of the solution. A buffer solution requires a weak acid/base conjugate pair, as opposed. How To Make Buffer More Acidic.
From www.youtube.com
How does and acidic buffer solution work? YouTube How To Make Buffer More Acidic To understand how buffers work, let’s look first at how the ionization equilibrium of a weak acid is affected by adding either the conjugate base of the acid or a strong acid (a source of \(h^+\)). Learn how buffers protect against ph changes when strong acid or base is added. Learn how to choose an acid/base pair and determine the. How To Make Buffer More Acidic.
From www.youtube.com
How to make acidic buffer solution? YouTube How To Make Buffer More Acidic It is able to neutralize small amounts of added acid or base, thus. A buffer solution is an aqueous solution that resists ph change. Learn how to choose an acid/base pair and determine the ratio of their concentrations to make a buffer solution with a given ph. Learn how buffers protect against ph changes when strong acid or base is. How To Make Buffer More Acidic.
From www.vrogue.co
How Buffers Work vrogue.co How To Make Buffer More Acidic Learn how buffers protect against ph changes when strong acid or base is added. A common example would be a mixture of ethanoic acid and sodium ethanoate in. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus.. How To Make Buffer More Acidic.
From www.osmosis.org
Making buffer solutions Osmosis How To Make Buffer More Acidic To understand how buffers work, let’s look first at how the ionization equilibrium of a weak acid is affected by adding either the conjugate base of the acid or a strong acid (a source of \(h^+\)). Learn how buffers protect against ph changes when strong acid or base is added. A buffer is a solution that can resist ph change. How To Make Buffer More Acidic.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint How To Make Buffer More Acidic How to excel at calculations for acidic buffers using the acid dissociation constant. Learn how to choose an acid/base pair and determine the ratio of their concentrations to make a buffer solution with a given ph. A common example would be a mixture of ethanoic acid and sodium ethanoate in. A mixture of a weak acid and its conjugate base. How To Make Buffer More Acidic.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high How To Make Buffer More Acidic A buffer solution is an aqueous solution that resists ph change. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Learn how to choose an acid/base pair and determine the ratio of their concentrations to make a buffer solution with a given. How To Make Buffer More Acidic.
From www.slideserve.com
PPT A solution that contains a weak acid/conjugate base mixture or How To Make Buffer More Acidic How to understand acidic buffers and their function. Le chatelier’s principle can be used to predict the effect on the equilibrium position of the solution. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. Learn how buffers protect against ph changes when. How To Make Buffer More Acidic.
From slideplayer.com
SCH4U Acids and Bases BUFFER SOLUTIONS. ppt download How To Make Buffer More Acidic A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer solution requires a weak acid/base conjugate pair, as opposed to a strong acid and its conjugate,. A buffer solution is an aqueous solution that resists ph change. To understand how buffers. How To Make Buffer More Acidic.
From criticalthinking.cloud
how to solve buffer problems chemistry How To Make Buffer More Acidic To understand how buffers work, let’s look first at how the ionization equilibrium of a weak acid is affected by adding either the conjugate base of the acid or a strong acid (a source of \(h^+\)). A buffer solution is an aqueous solution that resists ph change. A common example would be a mixture of ethanoic acid and sodium ethanoate. How To Make Buffer More Acidic.
From www.youtube.com
Buffer Solutions YouTube How To Make Buffer More Acidic It is able to neutralize small amounts of added acid or base, thus. Le chatelier’s principle can be used to predict the effect on the equilibrium position of the solution. Learn how to choose an acid/base pair and determine the ratio of their concentrations to make a buffer solution with a given ph. A buffer solution requires a weak acid/base. How To Make Buffer More Acidic.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint How To Make Buffer More Acidic It is able to neutralize small amounts of added acid or base, thus. Learn how buffers protect against ph changes when strong acid or base is added. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A mixture of a weak acid and its conjugate base (or a mixture of. How To Make Buffer More Acidic.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs How To Make Buffer More Acidic A buffer solution is an aqueous solution that resists ph change. A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or. A buffer solution requires a weak acid/base conjugate pair, as opposed to a strong acid and its conjugate,. Learn how to choose. How To Make Buffer More Acidic.