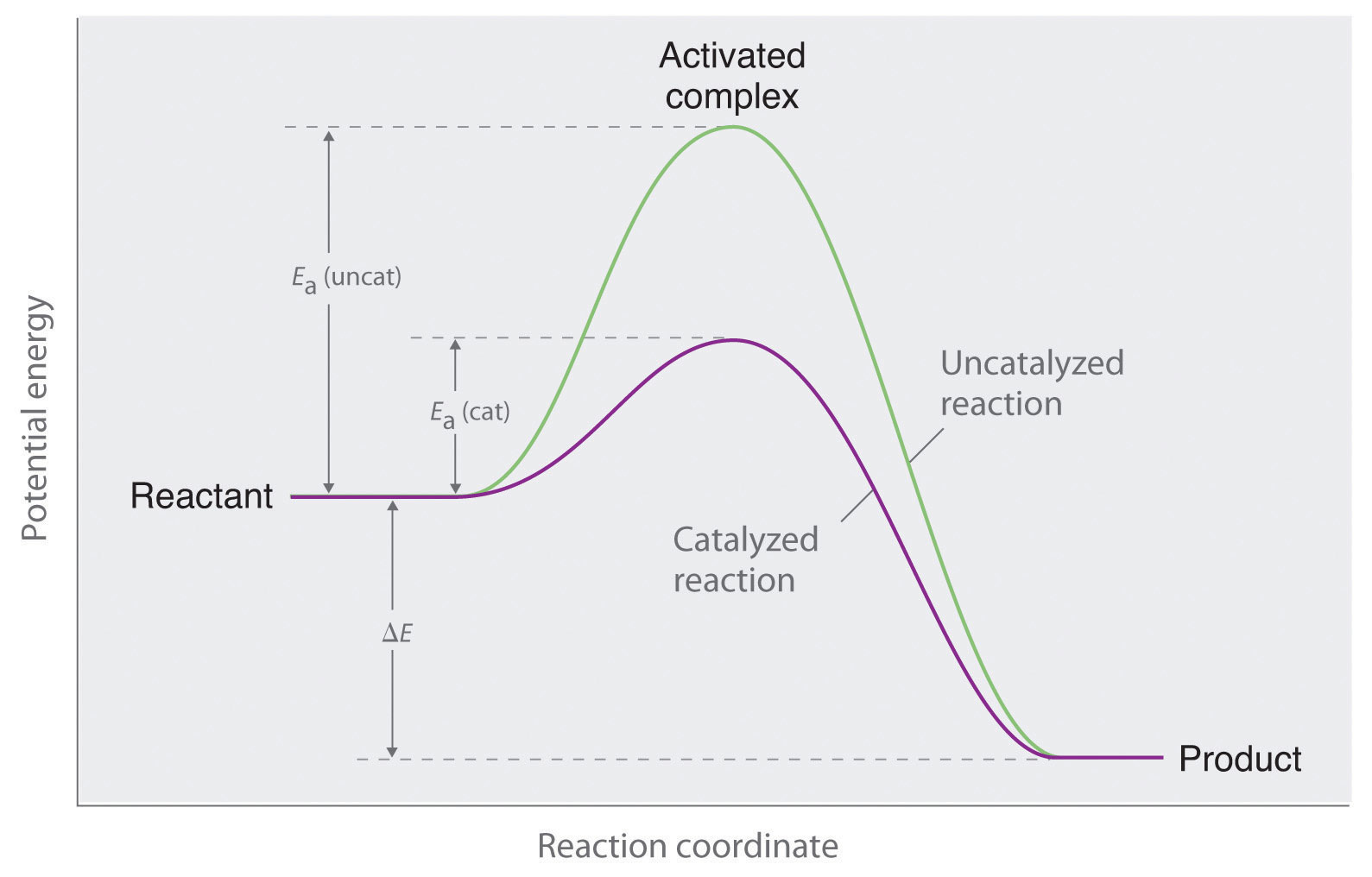
What Do Catalysts Do In Chemical Reactions . A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst, therefore, does not appear in the overall. It can also remove formaldehyde from the air. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. Enzymes are naturally occurring catalysts responsible for many.
from 2012books.lardbucket.org
A catalyst, therefore, does not appear in the overall. Enzymes are naturally occurring catalysts responsible for many. It can also remove formaldehyde from the air. Describe the similarities and differences between the. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. What are catalysts, and how do they work in terms altering the parameters of a reaction?
Catalysis
What Do Catalysts Do In Chemical Reactions A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst, therefore, does not appear in the overall. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. It can also remove formaldehyde from the air. Describe the similarities and differences between the. Enzymes are naturally occurring catalysts responsible for many.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism What Do Catalysts Do In Chemical Reactions Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. It can also remove formaldehyde from the air. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. Describe the similarities and differences between the. Catalyst, in chemistry, any substance. What Do Catalysts Do In Chemical Reactions.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts What Do Catalysts Do In Chemical Reactions What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without. What Do Catalysts Do In Chemical Reactions.
From www.youtube.com
How does a CATALYST work ? YouTube What Do Catalysts Do In Chemical Reactions Describe the similarities and differences between the. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed. What Do Catalysts Do In Chemical Reactions.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 What Do Catalysts Do In Chemical Reactions A catalyst, therefore, does not appear in the overall. It can also remove formaldehyde from the air. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is. What Do Catalysts Do In Chemical Reactions.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica What Do Catalysts Do In Chemical Reactions Enzymes are naturally occurring catalysts responsible for many. A catalyst, therefore, does not appear in the overall. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process.. What Do Catalysts Do In Chemical Reactions.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free What Do Catalysts Do In Chemical Reactions Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst, therefore, does not appear in the overall. What are catalysts, and how do they work in terms altering the parameters of a reaction? It. What Do Catalysts Do In Chemical Reactions.
From www.expii.com
What Is a Chemical Reaction? — Overview & Examples Expii What Do Catalysts Do In Chemical Reactions A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. A catalyst, therefore, does not appear in the overall. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts are substances that. What Do Catalysts Do In Chemical Reactions.
From vdocuments.mx
Catalysts Catalystlowers activation energy of a chemical reaction and What Do Catalysts Do In Chemical Reactions Describe the similarities and differences between the. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. A catalyst, therefore, does not appear. What Do Catalysts Do In Chemical Reactions.
From www.slideserve.com
PPT Role of Catalysts PowerPoint Presentation, free download ID2823169 What Do Catalysts Do In Chemical Reactions Describe the similarities and differences between the. Enzymes are naturally occurring catalysts responsible for many. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction. What Do Catalysts Do In Chemical Reactions.
From www.chemistrystudent.com
Catalysts (ALevel) ChemistryStudent What Do Catalysts Do In Chemical Reactions Describe the similarities and differences between the. Enzymes are naturally occurring catalysts responsible for many. A catalyst, therefore, does not appear in the overall. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalyst, in chemistry, any substance that increases the rate of a. What Do Catalysts Do In Chemical Reactions.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube What Do Catalysts Do In Chemical Reactions A catalyst, therefore, does not appear in the overall. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. Describe the similarities and differences between the. Enzymes are naturally occurring catalysts responsible for many. A catalyst is a chemical substance that affects the rate of a chemical reaction. What Do Catalysts Do In Chemical Reactions.
From www.pinterest.com
Catalyst and Mechanism of Enzyme Catalysis Enzymes, Chemistry, Active What Do Catalysts Do In Chemical Reactions A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst, therefore, does not appear in the overall. Enzymes are naturally occurring. What Do Catalysts Do In Chemical Reactions.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B What Do Catalysts Do In Chemical Reactions Describe the similarities and differences between the. It can also remove formaldehyde from the air. A catalyst, therefore, does not appear in the overall. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. Enzymes are naturally occurring catalysts responsible for many. A catalyst is a chemical substance that affects the rate. What Do Catalysts Do In Chemical Reactions.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions What Do Catalysts Do In Chemical Reactions Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Enzymes are naturally occurring catalysts responsible for many. A catalyst, therefore, does not appear in the overall.. What Do Catalysts Do In Chemical Reactions.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii What Do Catalysts Do In Chemical Reactions What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. It can also remove formaldehyde from. What Do Catalysts Do In Chemical Reactions.
From www.slideserve.com
PPT Basic Chemistry Concepts PowerPoint Presentation, free download What Do Catalysts Do In Chemical Reactions What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst, therefore, does not appear in the overall. Describe. What Do Catalysts Do In Chemical Reactions.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation What Do Catalysts Do In Chemical Reactions A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. It can also remove formaldehyde from the air. Enzymes are naturally occurring catalysts responsible for many. Describe the similarities and differences between the. A catalyst is a chemical substance added to a reaction to either. What Do Catalysts Do In Chemical Reactions.
From www.youtube.com
Identifying catalysts in a reaction YouTube What Do Catalysts Do In Chemical Reactions Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many. Describe the similarities and differences between the. It can also remove formaldehyde from the air. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is. What Do Catalysts Do In Chemical Reactions.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free What Do Catalysts Do In Chemical Reactions Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst, therefore,. What Do Catalysts Do In Chemical Reactions.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent What Do Catalysts Do In Chemical Reactions A catalyst, therefore, does not appear in the overall. What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is a chemical substance added to a reaction. What Do Catalysts Do In Chemical Reactions.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram What Do Catalysts Do In Chemical Reactions A catalyst, therefore, does not appear in the overall. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. Enzymes are naturally occurring catalysts responsible for many. Describe the similarities and differences. What Do Catalysts Do In Chemical Reactions.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation What Do Catalysts Do In Chemical Reactions What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. Catalyst,. What Do Catalysts Do In Chemical Reactions.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog What Do Catalysts Do In Chemical Reactions It can also remove formaldehyde from the air. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. A catalyst, therefore, does not appear. What Do Catalysts Do In Chemical Reactions.
From colouremployer8.gitlab.io
Supreme 4 Types Of Chemical Reactions Reactants And Products Cellular What Do Catalysts Do In Chemical Reactions A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst, therefore, does not appear in the overall. Catalysts are substances that increase the reaction rate of a chemical reaction without. What Do Catalysts Do In Chemical Reactions.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy What Do Catalysts Do In Chemical Reactions It can also remove formaldehyde from the air. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst, therefore, does not appear in the overall. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate. What Do Catalysts Do In Chemical Reactions.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub What Do Catalysts Do In Chemical Reactions It can also remove formaldehyde from the air. A catalyst, therefore, does not appear in the overall. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. Describe the similarities and differences between the. Enzymes are naturally occurring catalysts responsible for many. What are catalysts, and how do. What Do Catalysts Do In Chemical Reactions.
From 2012books.lardbucket.org
Catalysis What Do Catalysts Do In Chemical Reactions A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Enzymes are naturally occurring catalysts responsible for many. It can also remove formaldehyde from the air. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts are substances. What Do Catalysts Do In Chemical Reactions.
From www.cheric.org
Chemical Reaction (Reaction rate) What Do Catalysts Do In Chemical Reactions A catalyst, therefore, does not appear in the overall. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance added to a reaction. What Do Catalysts Do In Chemical Reactions.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis What Do Catalysts Do In Chemical Reactions Describe the similarities and differences between the. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. It can also remove formaldehyde from the air. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a chemical substance added to a reaction to. What Do Catalysts Do In Chemical Reactions.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of What Do Catalysts Do In Chemical Reactions Enzymes are naturally occurring catalysts responsible for many. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Describe the similarities and differences between the. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are substances that. What Do Catalysts Do In Chemical Reactions.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of What Do Catalysts Do In Chemical Reactions A catalyst, therefore, does not appear in the overall. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Enzymes are naturally occurring. What Do Catalysts Do In Chemical Reactions.
From www.waca.msf.org
Lesson Explainer Catalysts, Catalyst What Do Catalysts Do In Chemical Reactions It can also remove formaldehyde from the air. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst, therefore, does not appear in the overall. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself undergoing any. What are catalysts, and how. What Do Catalysts Do In Chemical Reactions.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube What Do Catalysts Do In Chemical Reactions What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the. Catalysts are substances that increase the reaction rate of a chemical reaction without being consumed in the process. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without. What Do Catalysts Do In Chemical Reactions.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) What Do Catalysts Do In Chemical Reactions A catalyst, therefore, does not appear in the overall. Describe the similarities and differences between the. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts are substances. What Do Catalysts Do In Chemical Reactions.
From www.slideserve.com
PPT Fast and Slow Chemistry PowerPoint Presentation, free download What Do Catalysts Do In Chemical Reactions Describe the similarities and differences between the. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst, therefore, does not appear in the overall. Enzymes are naturally occurring catalysts responsible for many. A catalyst is a chemical substance added to a reaction to either accelerate or decelerate the reaction rate without itself. What Do Catalysts Do In Chemical Reactions.