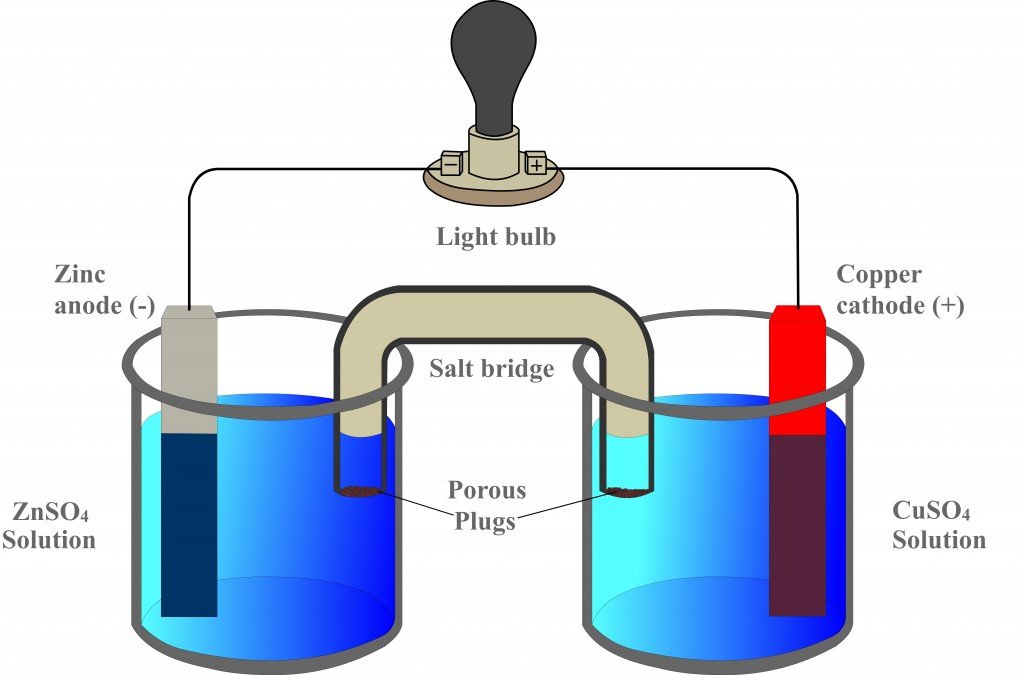
A Galvanic Cell In Chemistry . a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. Another common name for galvanic cells is. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox.
from www.scienceabc.com
Another common name for galvanic cells is. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current.
Galvanic Cell Definition, Diagram And Working
A Galvanic Cell In Chemistry a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. Another common name for galvanic cells is.
From biochemreview.weebly.com
Galvanic cell BIOChemReview A Galvanic Cell In Chemistry Another common name for galvanic cells is. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. A Galvanic Cell In Chemistry.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps A Galvanic Cell In Chemistry Another common name for galvanic cells is. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.
From thechemistrynotes.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle A Galvanic Cell In Chemistry a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. Another common name for galvanic cells is. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.
From ar.inspiredpencil.com
Galvanic Cell Labeled A Galvanic Cell In Chemistry Another common name for galvanic cells is. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps A Galvanic Cell In Chemistry a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. Another common name for galvanic cells is. A Galvanic Cell In Chemistry.
From chemistry.stackexchange.com
electrochemistry Galvanic cells/redox confused regarding diagram A Galvanic Cell In Chemistry Another common name for galvanic cells is. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. A Galvanic Cell In Chemistry.
From chem.libretexts.org
19.6 Commercial Galvanic Cells Chemistry LibreTexts A Galvanic Cell In Chemistry a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. Another common name for galvanic cells is. A Galvanic Cell In Chemistry.
From thechemistrynotes.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle A Galvanic Cell In Chemistry a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. Another common name for galvanic cells is. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.
From learningschoolletopisaln.z22.web.core.windows.net
Electrochemical Cells In Chemistry A Galvanic Cell In Chemistry a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. Another common name for galvanic cells is. A Galvanic Cell In Chemistry.
From www.myxxgirl.com
Chem Formal Lab Galvanic Cell Analysis Using Spontaneous Flowing My A Galvanic Cell In Chemistry an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. Another common name for galvanic cells is. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. A Galvanic Cell In Chemistry.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working A Galvanic Cell In Chemistry a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. Another common name for galvanic cells is. A Galvanic Cell In Chemistry.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic A Galvanic Cell In Chemistry a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. Another common name for galvanic cells is. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.
From glossary.periodni.com
Galvanic cell Chemistry Dictionary & Glossary A Galvanic Cell In Chemistry a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. Another common name for galvanic cells is. A Galvanic Cell In Chemistry.
From ar.inspiredpencil.com
Galvanic Cell Labeled A Galvanic Cell In Chemistry an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. Another common name for galvanic cells is. A Galvanic Cell In Chemistry.
From ar.inspiredpencil.com
Galvanic Cell A Galvanic Cell In Chemistry a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. Another common name for galvanic cells is. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.
From www.pinterest.co.uk
What are the differences between Galvanic cell and Electrolytic cell A Galvanic Cell In Chemistry an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. Another common name for galvanic cells is. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. A Galvanic Cell In Chemistry.
From psu.pb.unizin.org
Galvanic Cells (17.2) Chemistry 112 (Chapters 1217 of OpenStax A Galvanic Cell In Chemistry Another common name for galvanic cells is. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.
From mavink.com
Galvanic Cell Labeled A Galvanic Cell In Chemistry an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. Another common name for galvanic cells is. A Galvanic Cell In Chemistry.
From www.chemicals.co.uk
Measuring The EMF Of An Electrochemical Cell The Chemistry Blog A Galvanic Cell In Chemistry an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. Another common name for galvanic cells is. A Galvanic Cell In Chemistry.
From chemistry.stackexchange.com
electrochemistry What's the source of electrical energy in galvanic A Galvanic Cell In Chemistry Another common name for galvanic cells is. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. A Galvanic Cell In Chemistry.
From library.fiveable.me
Galvanic Cells Intro to Chemistry Study Guide 2024 Fiveable A Galvanic Cell In Chemistry an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. Another common name for galvanic cells is. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. A Galvanic Cell In Chemistry.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry A Galvanic Cell In Chemistry an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. Another common name for galvanic cells is. A Galvanic Cell In Chemistry.
From diagramdatablashiest.z5.web.core.windows.net
Electrochemical Cell Diagram A Galvanic Cell In Chemistry a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. Another common name for galvanic cells is. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle A Galvanic Cell In Chemistry an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. Another common name for galvanic cells is. A Galvanic Cell In Chemistry.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts A Galvanic Cell In Chemistry Another common name for galvanic cells is. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.
From www.vrogue.co
A Galvanic Cell Is Powered By The Following Redox Rea vrogue.co A Galvanic Cell In Chemistry a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. Another common name for galvanic cells is. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.
From www.chemistrylearner.com
Galvanic Cell (Voltaic Cell) Chemistry Learner A Galvanic Cell In Chemistry an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. Another common name for galvanic cells is. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. A Galvanic Cell In Chemistry.
From chem.libretexts.org
8.3 Galvanic Cells Chemistry LibreTexts A Galvanic Cell In Chemistry Another common name for galvanic cells is. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. A Galvanic Cell In Chemistry.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working A Galvanic Cell In Chemistry an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. Another common name for galvanic cells is. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. A Galvanic Cell In Chemistry.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE A Galvanic Cell In Chemistry Another common name for galvanic cells is. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.
From kemilektioner.se
Test Galvaniska element A Galvanic Cell In Chemistry Another common name for galvanic cells is. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.
From www.bigstockphoto.com
Voltaic Galvanic Cell Image & Photo (Free Trial) Bigstock A Galvanic Cell In Chemistry Another common name for galvanic cells is. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.
From in.pinterest.com
Understanding Galvanic Cells A Simplified Guide A Galvanic Cell In Chemistry an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. Another common name for galvanic cells is. A Galvanic Cell In Chemistry.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell A Galvanic Cell In Chemistry Another common name for galvanic cells is. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.
From www.tes.com
Galvanic Cells, Anode, Cathode, Oxidation, Reduction, Grade 12 A Galvanic Cell In Chemistry Another common name for galvanic cells is. a galvanic cell uses the energy released from a spontaneous redox reaction to produce an electric current. an apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox. A Galvanic Cell In Chemistry.