Hard Water Ka Formula . Water that does not produce lather with soap solution readily is known as hard water. Hard water is a term that denotes water having a very high mineral content (the term is the opposite. Hard water contains dissolved magnesium. what is hard water? the water in some parts of the country is soft, while the water in other parts of the country is hard. what is the formula for hard water? ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids.
from www.chegg.com
what is the formula for hard water? ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical. Hard water contains dissolved magnesium. what is hard water? Water that does not produce lather with soap solution readily is known as hard water. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. the water in some parts of the country is soft, while the water in other parts of the country is hard. Hard water is a term that denotes water having a very high mineral content (the term is the opposite. the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids.
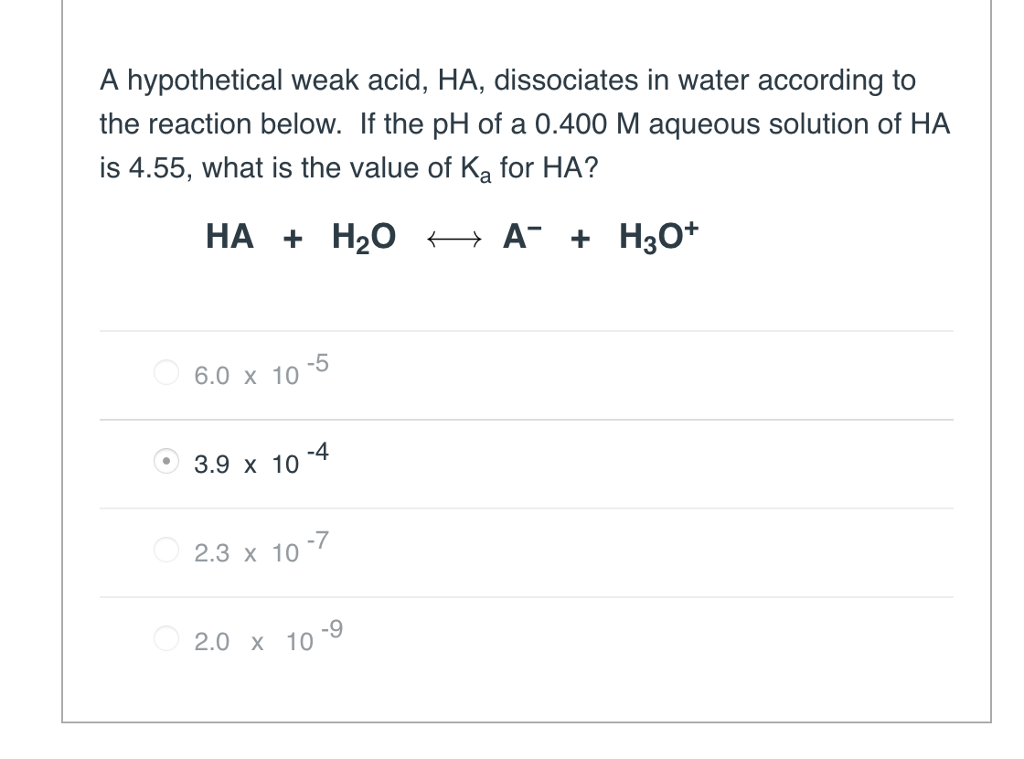
Solved A hypothetical weak acid, HA, dissociates in water
Hard Water Ka Formula hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. Water that does not produce lather with soap solution readily is known as hard water. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. Hard water contains dissolved magnesium. what is the formula for hard water? Hard water is a term that denotes water having a very high mineral content (the term is the opposite. what is hard water? the water in some parts of the country is soft, while the water in other parts of the country is hard. the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids. ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical.
From sciencenotes.org
What Is pKa in Chemistry? Acid Dissociation Constant Hard Water Ka Formula hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. Hard water contains dissolved magnesium. the water in some parts of the country is soft, while the water in other parts of the country is hard. the magnitude of the equilibrium constant for an ionization reaction can be used to determine. Hard Water Ka Formula.
From answerdbpersists.z13.web.core.windows.net
How To Solve For Ka Hard Water Ka Formula Hard water is a term that denotes water having a very high mineral content (the term is the opposite. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. what is the formula for hard water? what is hard water? Hard water contains dissolved magnesium. ph ≈ − log[h3o+] (1). Hard Water Ka Formula.
From oneclass.com
OneClass Write the balanced chemical equation for the reaction of the Hard Water Ka Formula the water in some parts of the country is soft, while the water in other parts of the country is hard. Hard water contains dissolved magnesium. what is hard water? hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. ph ≈ − log[h3o+] (1) or ph ≈ − log[h+]. Hard Water Ka Formula.
From jackwestin.com
Equilibrium Constants Ka And Kb Pka Pkb Acid Base Equilibria MCAT Hard Water Ka Formula what is hard water? the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. the water in some parts of the country is soft, while the water in other parts. Hard Water Ka Formula.
From netsolwater.com
Describe Of Hard Water In The Terms Of Mathematics Hard Water Ka Formula what is the formula for hard water? what is hard water? Hard water contains dissolved magnesium. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. the water in some parts of the country is soft, while the water in other parts of the country is hard. ph ≈. Hard Water Ka Formula.
From www.sliderbase.com
Acids, pH and Equilibrium Presentation Chemistry Hard Water Ka Formula Hard water is a term that denotes water having a very high mineral content (the term is the opposite. what is the formula for hard water? hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. Hard water contains dissolved magnesium. the water in some parts of the country is soft,. Hard Water Ka Formula.
From www.mspurelife.com
Hard Water VS Soft Water Is Hard or Soft Water Better for You Hard Water Ka Formula Water that does not produce lather with soap solution readily is known as hard water. Hard water is a term that denotes water having a very high mineral content (the term is the opposite. ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical. the magnitude. Hard Water Ka Formula.
From www.youtube.com
Strong Acid Ka Dissociation Constant Chemistry (HCl, HBr, HI, HNO3 Hard Water Ka Formula Hard water contains dissolved magnesium. the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids. ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical. Hard water is a term that denotes water having a very. Hard Water Ka Formula.
From jackwestin.com
Equilibrium Constants Ka And Kb Pka Pkb Acid Base Equilibria MCAT Hard Water Ka Formula hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. Water that does not produce lather with soap solution readily is known as hard water. the water in some parts of the country is soft, while the water in other parts of the country is hard. Hard water contains dissolved magnesium. . Hard Water Ka Formula.
From www.waterscience.in
What is Hard Water? WaterScience Hard Water Ka Formula Hard water contains dissolved magnesium. what is hard water? the water in some parts of the country is soft, while the water in other parts of the country is hard. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. what is the formula for hard water? ph ≈. Hard Water Ka Formula.
From a-z-animals.com
Hard Water vs. Soft Water Key Differences and How to Test Your Water Hard Water Ka Formula ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical. the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids. the water in some parts of the country is soft, while the water in other. Hard Water Ka Formula.
From www.tessshebaylo.com
Dissociation Of Acetic Acid In Water Equation Tessshebaylo Hard Water Ka Formula the water in some parts of the country is soft, while the water in other parts of the country is hard. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant. Hard Water Ka Formula.
From www.youtube.com
water ka formula kya hota hai?? water ka formula क्या होता है?? hme Hard Water Ka Formula Water that does not produce lather with soap solution readily is known as hard water. Hard water is a term that denotes water having a very high mineral content (the term is the opposite. the water in some parts of the country is soft, while the water in other parts of the country is hard. Hard water contains dissolved. Hard Water Ka Formula.
From www.numerade.com
Write the balanced Ka and Kb reactions for HSO3 in water. Be sure to Hard Water Ka Formula ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of. Hard Water Ka Formula.
From www.youtube.com
water ka formula। water ka formula janti ho? comedy water shorts Hard Water Ka Formula ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical. the water in some parts of the country is soft, while the water in other parts of the country is hard. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides,. Hard Water Ka Formula.
From wiener.me
What Is Value Of Ka And Kb For Water (H20) And How, 56 OFF Hard Water Ka Formula what is the formula for hard water? Hard water is a term that denotes water having a very high mineral content (the term is the opposite. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. Hard water contains dissolved magnesium. what is hard water? ph ≈ − log[h3o+] (1). Hard Water Ka Formula.
From ar.inspiredpencil.com
Ka Values And Acids Hard Water Ka Formula ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical. the water in some parts of the country is soft, while the water in other parts of the country is hard. Water that does not produce lather with soap solution readily is known as hard water.. Hard Water Ka Formula.
From sciencenotes.org
pH, pKa, Ka, pKb, and Kb in Chemistry Hard Water Ka Formula what is the formula for hard water? Water that does not produce lather with soap solution readily is known as hard water. ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical. Hard water contains dissolved magnesium. hard water, water that contains salts of calcium. Hard Water Ka Formula.
From learningchemistryeasily.blogspot.com
Learning Chemistry Easily Chemical Equilibrium Theory, Part 2 The Hard Water Ka Formula hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. Water that does not produce lather with soap solution readily is known as hard water. the water in some parts of the country is soft, while the water in other parts of the country is hard. Hard water is a term that. Hard Water Ka Formula.
From ar.inspiredpencil.com
Ka Values And Acids Hard Water Ka Formula hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids. what is hard water? Hard water is a term that denotes water having a very high mineral content (the term is. Hard Water Ka Formula.
From www.youtube.com
How To Calculate Ka Of An Acid From pH ? (with an Example) YouTube Hard Water Ka Formula Hard water is a term that denotes water having a very high mineral content (the term is the opposite. what is the formula for hard water? the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids. the water in some parts of the country is soft, while. Hard Water Ka Formula.
From www.numerade.com
SOLVED 'Write the acid dissociation and equilibrium expression (Ka Hard Water Ka Formula the water in some parts of the country is soft, while the water in other parts of the country is hard. the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids. ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant,. Hard Water Ka Formula.
From www.youtube.com
AP Chemistry Notes 8.5 Calculations with Ka YouTube Hard Water Ka Formula what is the formula for hard water? Hard water is a term that denotes water having a very high mineral content (the term is the opposite. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. Hard water contains dissolved magnesium. the magnitude of the equilibrium constant for an ionization reaction. Hard Water Ka Formula.
From ar.inspiredpencil.com
Ka Values And Acids Hard Water Ka Formula hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids. what is hard water? Hard water contains dissolved magnesium. Water that does not produce lather with soap solution readily is known. Hard Water Ka Formula.
From www.diplomageeks.com
Define soft water and hard water Hard Water Ka Formula what is the formula for hard water? ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical. the water in some parts of the country is soft, while the water in other parts of the country is hard. hard water, water that contains salts. Hard Water Ka Formula.
From www.nagwa.com
Question Video Writing an Equation for the Acid Dissociation Constant Hard Water Ka Formula ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical. the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids. the water in some parts of the country is soft, while the water in other. Hard Water Ka Formula.
From www.slideserve.com
PPT Ka, Kb PowerPoint Presentation, free download ID6823732 Hard Water Ka Formula the water in some parts of the country is soft, while the water in other parts of the country is hard. what is the formula for hard water? Hard water is a term that denotes water having a very high mineral content (the term is the opposite. hard water, water that contains salts of calcium and magnesium. Hard Water Ka Formula.
From barkmanoil.com
Water Ka Value? Best 23 Answer Hard Water Ka Formula what is hard water? hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. what is the formula for hard water? the water in some parts of the country is soft, while the water in other parts of the country is hard. Hard water is a term that denotes water. Hard Water Ka Formula.
From madelynmeowstafford.blogspot.com
Calculate the Acid Ionization Constant Ka for the Acid Hard Water Ka Formula what is hard water? the water in some parts of the country is soft, while the water in other parts of the country is hard. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. Hard water contains dissolved magnesium. the magnitude of the equilibrium constant for an ionization reaction. Hard Water Ka Formula.
From www.masterorganicchemistry.com
How To Use a pKa Table Hard Water Ka Formula what is hard water? Hard water contains dissolved magnesium. what is the formula for hard water? the water in some parts of the country is soft, while the water in other parts of the country is hard. Hard water is a term that denotes water having a very high mineral content (the term is the opposite. . Hard Water Ka Formula.
From www.chegg.com
Solved A hypothetical weak acid, HA, dissociates in water Hard Water Ka Formula what is the formula for hard water? ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical. the water in some parts of the country is soft, while the water in other parts of the country is hard. the magnitude of the equilibrium constant. Hard Water Ka Formula.
From www.tessshebaylo.com
Ionization Of Acetic Acid Equilibrium Equation Tessshebaylo Hard Water Ka Formula Water that does not produce lather with soap solution readily is known as hard water. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. the magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids. what is hard water? ph. Hard Water Ka Formula.
From brainly.com
Write a balanced equation and Ka expression for the BrønstedLowry acid Hard Water Ka Formula the water in some parts of the country is soft, while the water in other parts of the country is hard. what is the formula for hard water? ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical. Hard water contains dissolved magnesium. the. Hard Water Ka Formula.
From www.youtube.com
18.2 State the equation for reaction of weak acid/base in water,and Hard Water Ka Formula ph ≈ − log[h3o+] (1) or ph ≈ − log[h+] (2) ka, the acid ionization constant, is the equilibrium constant for chemical. what is hard water? the water in some parts of the country is soft, while the water in other parts of the country is hard. what is the formula for hard water? Water that. Hard Water Ka Formula.
From chemistry.stackovernet.xyz
homework 왜 Ka (HCN)가 주어 졌을 때 NaCN의 pH를 찾기 위해 3 개의 방정식이 필요합니까? Hard Water Ka Formula the water in some parts of the country is soft, while the water in other parts of the country is hard. hard water, water that contains salts of calcium and magnesium principally as bicarbonates, chlorides, and sulfates. Hard water is a term that denotes water having a very high mineral content (the term is the opposite. Water that. Hard Water Ka Formula.