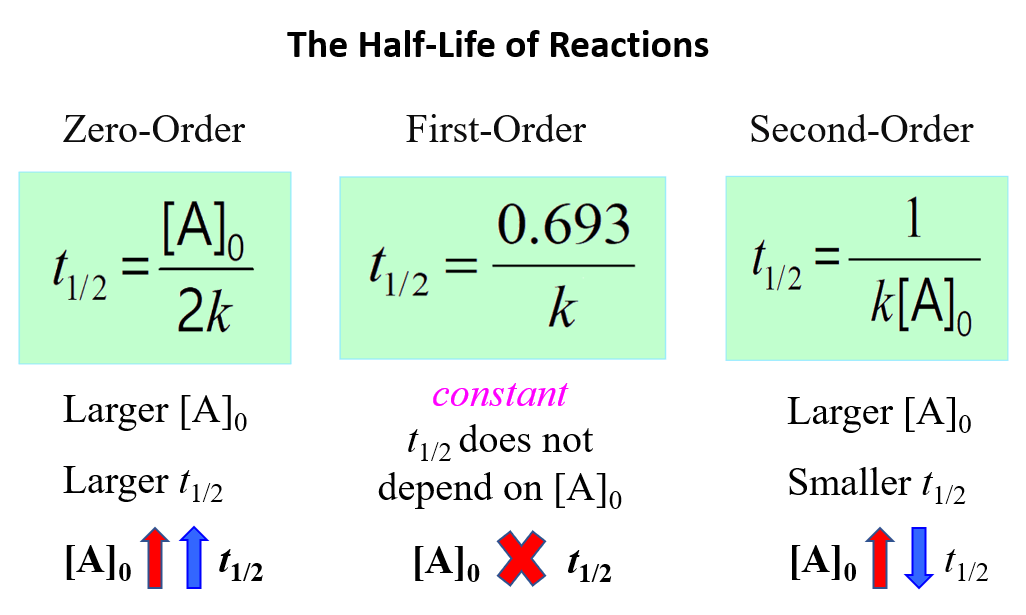
The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is . A certain first order reaction is 45.0% complete in 65 s. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. The order of the reaction or enough. Ethyl chloride decomposes to ethylene and hcl in a first.
from general.chemistrysteps.com
A certain first order reaction is 45.0% complete in 65 s. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Ethyl chloride decomposes to ethylene and hcl in a first. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. The order of the reaction or enough.
HalfLife of a Reaction Chemistry Steps
The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is Ethyl chloride decomposes to ethylene and hcl in a first. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. The order of the reaction or enough. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. Ethyl chloride decomposes to ethylene and hcl in a first. A certain first order reaction is 45.0% complete in 65 s.
From www.numerade.com
SOLVED Halflife equation for firstorder reactions t1/2 = 0.693/k The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is Ethyl chloride decomposes to ethylene and hcl in a first. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. A certain first order reaction. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.showme.com
Finding halflife for a first order reaction Science, Chemistry The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is A certain first order reaction is 45.0% complete in 65 s. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. Ethyl chloride decomposes to. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From mungfali.com
First Order Half Life Graph The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Ethyl chloride decomposes to ethylene and hcl in a first. The order of the reaction or enough. A certain first order reaction is 45.0% complete in 65 s. Assuming we have the same data, time and the concentrations of a. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From chemistryguru.com.sg
Rate Equation and Order of Reaction The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is A certain first order reaction is 45.0% complete in 65 s. The order of the reaction or enough. Ethyl chloride decomposes to ethylene and hcl in a first. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. We can figure out the half life. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From askfilo.com
For a first order reaction A→B, the rate constant, k=5.5×10−14 s−1. The t.. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Ethyl chloride decomposes to ethylene and hcl in a first. A certain first order reaction is 45.0% complete in 65 s. The order of the reaction or enough. Assuming we have the same data, time and the concentrations of a. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From ar.inspiredpencil.com
First Order Reaction Rate The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is The order of the reaction or enough. Ethyl chloride decomposes to ethylene and hcl in a first. A certain first order reaction is 45.0% complete in 65 s. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. We can figure out the half life. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.youtube.com
The rate constant for a first order reaction six times when the The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is Ethyl chloride decomposes to ethylene and hcl in a first. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. The order of the reaction. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.youtube.com
Determine the halflife of a first order reaction YouTube The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. Ethyl chloride decomposes to ethylene and hcl in a first. A certain first order reaction is 45.0% complete in 65 s. We can figure out the half life for a first order reaction from a. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From byjus.com
How to calculate the half life of a first order reaction The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is The order of the reaction or enough. Ethyl chloride decomposes to ethylene and hcl in a first. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. A certain first order reaction is 45.0% complete in 65 s. Assuming we have the same data, time and the concentrations of a. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. The order of the reaction or enough. A certain first order reaction is 45.0% complete. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From chem.libretexts.org
4.5 First Order Reaction HalfLife Chemistry LibreTexts The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is The order of the reaction or enough. Ethyl chloride decomposes to ethylene and hcl in a first. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. A certain first order reaction is 45.0% complete in 65 s. We can figure out the half life. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.brainkart.com
Rate equation for first order reactions The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is The order of the reaction or enough. A certain first order reaction is 45.0% complete in 65 s. Ethyl chloride decomposes to ethylene and hcl in a first. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. We can figure out the half life. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From socratic.org
What is a first order half life? Socratic The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. The order of the reaction or enough. Ethyl chloride decomposes to ethylene and hcl in a first. A certain first order reaction is 45.0% complete in 65 s. We can figure out the half life. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.researchgate.net
How to calculate rate constant for first order reaction? ResearchGate The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is A certain first order reaction is 45.0% complete in 65 s. Ethyl chloride decomposes to ethylene and hcl in a first. The order of the reaction or enough. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Assuming we have the same data, time and the concentrations of a. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.youtube.com
First Order Reactions Chemistry Class 12 IIT JEE Main + Advanced The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is A certain first order reaction is 45.0% complete in 65 s. Ethyl chloride decomposes to ethylene and hcl in a first. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. The order of the reaction or enough. We can figure out the half life. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From study.com
Solving for FirstOrder given HalfLife Chemistry The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. A certain first order reaction is 45.0% complete in 65 s. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. The order of the. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.toppr.com
The half life of a first order reaction is 480s . Then, the rate The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. The order of the reaction or enough. Ethyl chloride decomposes to ethylene and hcl in a first. A certain first order reaction is 45.0% complete in 65 s. We can figure out the half life. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.tessshebaylo.com
Derive An Integrated Rate Equation For Constant The First Order The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is The order of the reaction or enough. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. Ethyl chloride decomposes to ethylene and hcl in a first. A certain first order reaction is 45.0% complete in 65 s. We can figure out the half life. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.youtube.com
First Order Elimination Rate Constant and Halflife A closer look The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is A certain first order reaction is 45.0% complete in 65 s. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Ethyl chloride decomposes to. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From chemistnotes.com
First Order Reaction definition, example, half life period Chemistry The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Ethyl chloride decomposes to ethylene and hcl in a first. The order of the reaction or enough. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.slideserve.com
PPT Chapter 15 Chemical The Rates of Chemical Reactions The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is We can figure out the half life for a first order reaction from a graph of [reactant] against time or. The order of the reaction or enough. Ethyl chloride decomposes to ethylene and hcl in a first. A certain first order reaction is 45.0% complete in 65 s. Assuming we have the same data, time and the concentrations of a. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From byjus.com
What is the unit of rate constant for first order reaction The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. Ethyl chloride decomposes to ethylene and hcl in a first. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. A certain first order reaction. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From general.chemistrysteps.com
HalfLife of a Reaction Chemistry Steps The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is The order of the reaction or enough. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. A certain first order reaction is 45.0% complete in 65 s. Ethyl chloride decomposes to ethylene and hcl in a first. Assuming we have the same data, time and the concentrations of a. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From trixalagom.blogspot.com
half life formula for first order reaction Burdensome Online Journal The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. A certain first order reaction is 45.0% complete in 65 s. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Ethyl chloride decomposes to. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From askfilo.com
The rate constant and halflife of a first order reaction are related to The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is The order of the reaction or enough. Ethyl chloride decomposes to ethylene and hcl in a first. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. We can figure out the half life for a first order reaction from a graph of [reactant] against. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.chegg.com
Solved A firstorder reaction has a halflife of 87.1 min at The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is The order of the reaction or enough. Ethyl chloride decomposes to ethylene and hcl in a first. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. We can figure out the half life for a first order reaction from a graph of [reactant] against. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.numerade.com
SOLVED '8) The halflife for a firstorder reaction is 32.0 Determine The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is A certain first order reaction is 45.0% complete in 65 s. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Ethyl chloride decomposes to ethylene and hcl in a first. The order of the reaction or enough. Assuming we have the same data, time and the concentrations of a. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.chegg.com
Solved What is the rate constant of a firstorder reaction The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Ethyl chloride decomposes to ethylene and hcl in a first. The order of the reaction or enough. A certain first order reaction is 45.0% complete in 65 s. Assuming we have the same data, time and the concentrations of a. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.slideserve.com
PPT Chemical Chapter 14 PowerPoint Presentation, free The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is The order of the reaction or enough. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Ethyl chloride decomposes to ethylene and hcl in a first. A certain first order reaction is 45.0% complete in 65 s. Assuming we have the same data, time and the concentrations of a. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From study.com
First Order Reaction & Rate Law Definition, Equation & Examples The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is We can figure out the half life for a first order reaction from a graph of [reactant] against time or. The order of the reaction or enough. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. Ethyl chloride decomposes to ethylene and hcl in. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From newbedev.com
Chemistry Formula for rate constant for the first order reaction The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is The order of the reaction or enough. A certain first order reaction is 45.0% complete in 65 s. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Ethyl chloride decomposes to ethylene and hcl in a first. Assuming we have the same data, time and the concentrations of a. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID5829521 The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is The order of the reaction or enough. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. Ethyl chloride decomposes to ethylene and hcl in. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.youtube.com
first order reaction rate calculation example YouTube The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. Ethyl chloride decomposes to ethylene and hcl in a first. A certain first order reaction is 45.0% complete in 65 s. We can figure out the half life for a first order reaction from a. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From www.youtube.com
Halflife of a firstorder reaction AP Chemistry Khan The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is Ethyl chloride decomposes to ethylene and hcl in a first. We can figure out the half life for a first order reaction from a graph of [reactant] against time or. A certain first order reaction is 45.0% complete in 65 s. The order of the reaction or enough. Assuming we have the same data, time and the concentrations of a. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.
From study.com
Identifying HalfLife Given the Rate Constant Chemistry The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is We can figure out the half life for a first order reaction from a graph of [reactant] against time or. Assuming we have the same data, time and the concentrations of a reactant, and that we know that the reaction is a first order reaction. A certain first order reaction is 45.0% complete in 65 s. Ethyl chloride decomposes to. The Rate Constant For A First Order Reaction Which Has Half Life 693 S Is.