What Does C Stand For In Thermodynamics . The specific heat is the amount of heat necessary to change the temperature. the symbol c stands for specific heat and depends on the material and phase. — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known as the. heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. The specific heat is the amount of heat necessary to change the temperature. the symbol \(c\) stands for specific heat and depends on the material and phase. \(c_p\) is always greater than \(c_v\), but. The unit of heat capacity is joule per kelvin or joule per degree celsius. 38 rows — thermodynamic properties are defined as characteristic features of a system, capable of specifying the. for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of temperature. Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property.
from www.slideserve.com
The specific heat is the amount of heat necessary to change the temperature. Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property. — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known as the. The unit of heat capacity is joule per kelvin or joule per degree celsius. 38 rows — thermodynamic properties are defined as characteristic features of a system, capable of specifying the. for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of temperature. the symbol c stands for specific heat and depends on the material and phase. \(c_p\) is always greater than \(c_v\), but. heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. The specific heat is the amount of heat necessary to change the temperature.
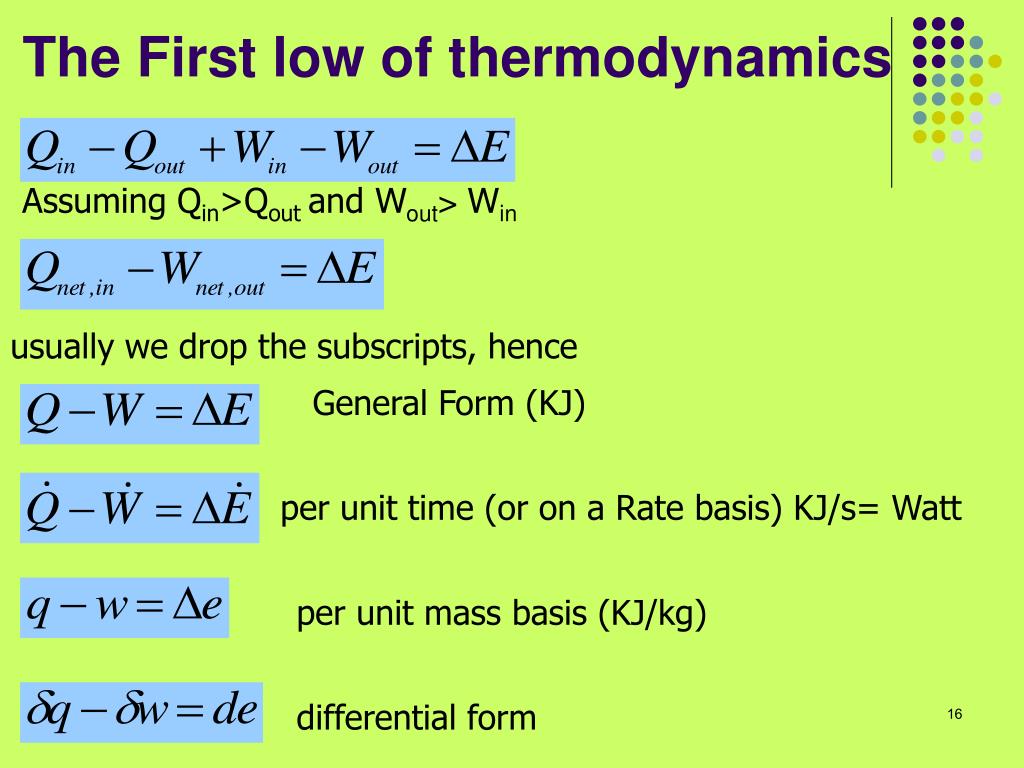
PPT The First Law of Thermodynamics PowerPoint Presentation, free
What Does C Stand For In Thermodynamics 38 rows — thermodynamic properties are defined as characteristic features of a system, capable of specifying the. the symbol \(c\) stands for specific heat and depends on the material and phase. The unit of heat capacity is joule per kelvin or joule per degree celsius. The specific heat is the amount of heat necessary to change the temperature. for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of temperature. \(c_p\) is always greater than \(c_v\), but. heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known as the. Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property. The specific heat is the amount of heat necessary to change the temperature. 38 rows — thermodynamic properties are defined as characteristic features of a system, capable of specifying the. the symbol c stands for specific heat and depends on the material and phase.
From circuitenginecawed88.z21.web.core.windows.net
Thermodynamics And Phase Diagrams What Does C Stand For In Thermodynamics Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property. for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of temperature. \(c_p\) is always greater than \(c_v\), but. the symbol \(c\) stands for specific heat and depends on the material and phase. heat capacity is defined. What Does C Stand For In Thermodynamics.
From www.youtube.com
Deriving the Thermodynamic Master Equations (Chemistry/Physics) YouTube What Does C Stand For In Thermodynamics the symbol c stands for specific heat and depends on the material and phase. — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known as the. The specific heat is the amount of heat necessary to change the temperature. The specific heat is the amount of heat necessary to. What Does C Stand For In Thermodynamics.
From www.studiobinder.com
What is a CStand — Types, Functions & Proper Usage Explained What Does C Stand For In Thermodynamics The specific heat is the amount of heat necessary to change the temperature. the symbol c stands for specific heat and depends on the material and phase. heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. The specific heat is the amount. What Does C Stand For In Thermodynamics.
From www.grc.nasa.gov
Conservation of Energy What Does C Stand For In Thermodynamics — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known as the. Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property. heat capacity is defined as the amount of heat energy required to raise the temperature of a given. What Does C Stand For In Thermodynamics.
From thechemistrynotes.com
Zeroth Law of Thermodynamics with Application and Examples What Does C Stand For In Thermodynamics \(c_p\) is always greater than \(c_v\), but. Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property. The unit of heat capacity is joule per kelvin or joule per degree celsius. — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known. What Does C Stand For In Thermodynamics.
From www.youtube.com
Thermodynamics 1 C2 L10 First law of thermodynamics Energy What Does C Stand For In Thermodynamics for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of temperature. The specific heat is the amount of heat necessary to change the temperature. the symbol \(c\) stands for specific heat and depends on the material and phase. 38 rows — thermodynamic properties are defined as characteristic features of a system, capable of specifying the.. What Does C Stand For In Thermodynamics.
From thechemistrynotes.com
Thermodynamic Processes The Chemistry Notes What Does C Stand For In Thermodynamics heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. The specific heat is the amount of heat necessary to change the temperature. The specific heat is the amount of heat necessary to change the temperature. the symbol c stands for specific heat. What Does C Stand For In Thermodynamics.
From www.chegg.com
Solved Appendix C Thermodynamic Data Table C.1 Standard E... What Does C Stand For In Thermodynamics \(c_p\) is always greater than \(c_v\), but. — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known as the. Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property. for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions. What Does C Stand For In Thermodynamics.
From www.pathwaystochemistry.com
Thermodynamic Quantities at 25°C Pathways to Chemistry What Does C Stand For In Thermodynamics \(c_p\) is always greater than \(c_v\), but. Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property. The unit of heat capacity is joule per kelvin or joule per degree celsius. The specific heat is the amount of heat necessary to change the temperature. heat capacity is defined as the. What Does C Stand For In Thermodynamics.
From www.slideshare.net
Thermodynamic cycles What Does C Stand For In Thermodynamics the symbol c stands for specific heat and depends on the material and phase. The specific heat is the amount of heat necessary to change the temperature. heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. — in thermodynamics, the heat. What Does C Stand For In Thermodynamics.
From www.youtube.com
The First Law of Thermodynamics Internal Energy, Heat, and Work YouTube What Does C Stand For In Thermodynamics the symbol c stands for specific heat and depends on the material and phase. The unit of heat capacity is joule per kelvin or joule per degree celsius. 38 rows — thermodynamic properties are defined as characteristic features of a system, capable of specifying the. for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of. What Does C Stand For In Thermodynamics.
From exozpkwqw.blob.core.windows.net
Forms Of Energy In Thermodynamics at Glennis Fluharty blog What Does C Stand For In Thermodynamics — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known as the. The specific heat is the amount of heat necessary to change the temperature. 38 rows — thermodynamic properties are defined as characteristic features of a system, capable of specifying the. \(c_p\) is always greater than \(c_v\), but.. What Does C Stand For In Thermodynamics.
From worksheetve1nadr.z13.web.core.windows.net
Thermodynamics 2 Equation Sheet What Does C Stand For In Thermodynamics The specific heat is the amount of heat necessary to change the temperature. for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of temperature. \(c_p\) is always greater than \(c_v\), but. the symbol c stands for specific heat and depends on the material and phase. 38 rows — thermodynamic properties are defined as characteristic features. What Does C Stand For In Thermodynamics.
From www.youtube.com
Thermodynamics (Physics) Lesson 2 Heat Transfer and Specific Heat.avi What Does C Stand For In Thermodynamics — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known as the. heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. \(c_p\) is always greater than \(c_v\), but. The specific heat is the. What Does C Stand For In Thermodynamics.
From lawofthermodynamicsinfo.com
4 Laws Of Thermodynamics With Examples (Very Simple) What Does C Stand For In Thermodynamics for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of temperature. the symbol c stands for specific heat and depends on the material and phase. Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property. The specific heat is the amount of heat necessary to change the. What Does C Stand For In Thermodynamics.
From www.slideserve.com
PPT The First Law of Thermodynamics PowerPoint Presentation, free What Does C Stand For In Thermodynamics 38 rows — thermodynamic properties are defined as characteristic features of a system, capable of specifying the. for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of temperature. \(c_p\) is always greater than \(c_v\), but. The unit of heat capacity is joule per kelvin or joule per degree celsius. The specific heat is the amount of. What Does C Stand For In Thermodynamics.
From www.slideserve.com
PPT Components of Thermodynamic Cycles PowerPoint Presentation ID What Does C Stand For In Thermodynamics The unit of heat capacity is joule per kelvin or joule per degree celsius. 38 rows — thermodynamic properties are defined as characteristic features of a system, capable of specifying the. Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property. — in thermodynamics, the heat capacity ratio or. What Does C Stand For In Thermodynamics.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID1230102 What Does C Stand For In Thermodynamics the symbol \(c\) stands for specific heat and depends on the material and phase. The specific heat is the amount of heat necessary to change the temperature. Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property. for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of. What Does C Stand For In Thermodynamics.
From www3.nd.edu
Thermodynamic units and constants What Does C Stand For In Thermodynamics The specific heat is the amount of heat necessary to change the temperature. — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known as the. \(c_p\) is always greater than \(c_v\), but. Heat capacity for a given matter depends on its size or quantity and hence it is an extensive. What Does C Stand For In Thermodynamics.
From www.geeksforgeeks.org
What is Thermodynamics Definition, Laws, Formulas, Class 11 Notes What Does C Stand For In Thermodynamics \(c_p\) is always greater than \(c_v\), but. The specific heat is the amount of heat necessary to change the temperature. the symbol c stands for specific heat and depends on the material and phase. The specific heat is the amount of heat necessary to change the temperature. Heat capacity for a given matter depends on its size or quantity. What Does C Stand For In Thermodynamics.
From www.youtube.com
5 2 2 The First Law of Thermodynamics YouTube What Does C Stand For In Thermodynamics The specific heat is the amount of heat necessary to change the temperature. 38 rows — thermodynamic properties are defined as characteristic features of a system, capable of specifying the. the symbol \(c\) stands for specific heat and depends on the material and phase. the symbol c stands for specific heat and depends on the material and. What Does C Stand For In Thermodynamics.
From www.youtube.com
First Law of Thermodynamics Thermal energy and Work Done YouTube What Does C Stand For In Thermodynamics for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of temperature. — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known as the. The unit of heat capacity is joule per kelvin or joule per degree celsius. Heat capacity for a given matter depends on its size. What Does C Stand For In Thermodynamics.
From www.slideshare.net
Thermodynamics What Does C Stand For In Thermodynamics — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known as the. for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of temperature. the symbol c stands for specific heat and depends on the material and phase. The specific heat is the amount of heat necessary. What Does C Stand For In Thermodynamics.
From thechemistrynotes.com
Thermodynamics and Laws of Thermodynamics What Does C Stand For In Thermodynamics the symbol c stands for specific heat and depends on the material and phase. The unit of heat capacity is joule per kelvin or joule per degree celsius. heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. — in thermodynamics, the. What Does C Stand For In Thermodynamics.
From present5.com
Chemical thermodynamics lesson plan 1 Types of What Does C Stand For In Thermodynamics heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of temperature. 38 rows — thermodynamic properties are defined as characteristic features of a system, capable of specifying the. The unit. What Does C Stand For In Thermodynamics.
From www.theengineerspost.com
3 Laws of Thermodynamics Explained with Examples PDF What Does C Stand For In Thermodynamics for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of temperature. the symbol \(c\) stands for specific heat and depends on the material and phase. — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known as the. heat capacity is defined as the amount of. What Does C Stand For In Thermodynamics.
From www.slideserve.com
PPT Thermodynamics I Chapter 2 Properties of Pure Substances What Does C Stand For In Thermodynamics the symbol c stands for specific heat and depends on the material and phase. the symbol \(c\) stands for specific heat and depends on the material and phase. The specific heat is the amount of heat necessary to change the temperature. heat capacity is defined as the amount of heat energy required to raise the temperature of. What Does C Stand For In Thermodynamics.
From www.slideserve.com
PPT Chapter 18 The Laws of Thermodynamics PowerPoint Presentation What Does C Stand For In Thermodynamics Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property. The specific heat is the amount of heat necessary to change the temperature. for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of temperature. The unit of heat capacity is joule per kelvin or joule per degree celsius.. What Does C Stand For In Thermodynamics.
From www.youtube.com
Different Types Of Thermodynamic Process Basic Concepts Engineering What Does C Stand For In Thermodynamics heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. the symbol c stands for specific heat and depends on the material and phase. The specific heat is the amount of heat necessary to change the temperature. for polyatomic gases, real or. What Does C Stand For In Thermodynamics.
From socratic.org
What are the three laws of thermodynamics? Socratic What Does C Stand For In Thermodynamics The unit of heat capacity is joule per kelvin or joule per degree celsius. The specific heat is the amount of heat necessary to change the temperature. — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known as the. the symbol \(c\) stands for specific heat and depends on. What Does C Stand For In Thermodynamics.
From www.eigenplus.com
Thermodynamic Process Definition, Types & Examples eigenplus What Does C Stand For In Thermodynamics the symbol c stands for specific heat and depends on the material and phase. The unit of heat capacity is joule per kelvin or joule per degree celsius. Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property. the symbol \(c\) stands for specific heat and depends on the. What Does C Stand For In Thermodynamics.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions What Does C Stand For In Thermodynamics — in thermodynamics, the heat capacity ratio or ratio of specific heat capacities (c p:c v) is also known as the. 38 rows — thermodynamic properties are defined as characteristic features of a system, capable of specifying the. The unit of heat capacity is joule per kelvin or joule per degree celsius. \(c_p\) is always greater than \(c_v\),. What Does C Stand For In Thermodynamics.
From physical-phenomena.blogspot.com
Laws of Thermodynamics What Does C Stand For In Thermodynamics Heat capacity for a given matter depends on its size or quantity and hence it is an extensive property. The specific heat is the amount of heat necessary to change the temperature. the symbol c stands for specific heat and depends on the material and phase. 38 rows — thermodynamic properties are defined as characteristic features of a. What Does C Stand For In Thermodynamics.
From justinfozone.blogspot.com
Thermodynamicsits system,laws and processes Informational Encyclopedia What Does C Stand For In Thermodynamics heat capacity is defined as the amount of heat energy required to raise the temperature of a given quantity of matter by one degree celsius. The specific heat is the amount of heat necessary to change the temperature. The specific heat is the amount of heat necessary to change the temperature. for polyatomic gases, real or ideal, \(c_v\). What Does C Stand For In Thermodynamics.
From thechemistrynotes.com
Third Law of Thermodynamics The Chemistry Notes What Does C Stand For In Thermodynamics The unit of heat capacity is joule per kelvin or joule per degree celsius. The specific heat is the amount of heat necessary to change the temperature. \(c_p\) is always greater than \(c_v\), but. for polyatomic gases, real or ideal, \(c_v\) and \(c_p\) are functions of temperature. heat capacity is defined as the amount of heat energy required. What Does C Stand For In Thermodynamics.