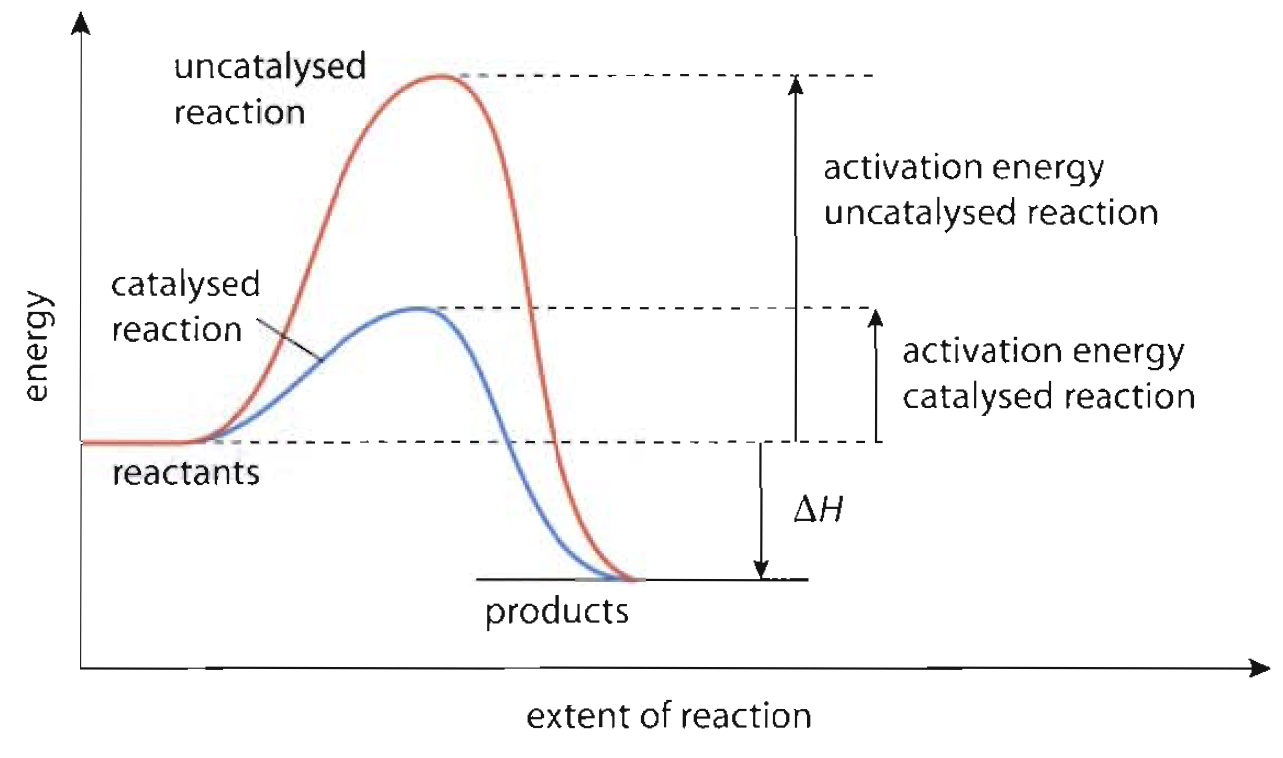
Why Do Catalysts Lower The Activation Energy . learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how activation energy is the energy required for a reaction to occur and determines its rate. See examples, diagrams and explanations of the. learn the fundamental principles of catalysis and how the mechanisms are studied with examples and diagrams. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. — activation energy is the minimum energy needed to start a chemical reaction. Find out how temperature affects the activation energy and the. Learn how to calculate it, how it affects reaction rate, and.
from sheetalschemblog.blogspot.com
learn the fundamental principles of catalysis and how the mechanisms are studied with examples and diagrams. Find out how temperature affects the activation energy and the. — activation energy is the minimum energy needed to start a chemical reaction. — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. See examples, diagrams and explanations of the. Learn how to calculate it, how it affects reaction rate, and. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how activation energy is the energy required for a reaction to occur and determines its rate. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed.
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7
Why Do Catalysts Lower The Activation Energy — activation energy is the minimum energy needed to start a chemical reaction. Find out how temperature affects the activation energy and the. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. learn how activation energy is the energy required for a reaction to occur and determines its rate. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn the fundamental principles of catalysis and how the mechanisms are studied with examples and diagrams. — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. — activation energy is the minimum energy needed to start a chemical reaction. Learn how to calculate it, how it affects reaction rate, and. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. See examples, diagrams and explanations of the.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Why Do Catalysts Lower The Activation Energy Find out how temperature affects the activation energy and the. learn how activation energy is the energy required for a reaction to occur and determines its rate. — activation energy is the minimum energy needed to start a chemical reaction. See examples, diagrams and explanations of the. catalysts make this process more efficient by lowering the activation. Why Do Catalysts Lower The Activation Energy.
From slideplayer.com
Activation Energy and Catalysts ppt download Why Do Catalysts Lower The Activation Energy See examples, diagrams and explanations of the. Find out how temperature affects the activation energy and the. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn the fundamental principles of catalysis and. Why Do Catalysts Lower The Activation Energy.
From www.slideserve.com
PPT Reaction Rate and Equilibrium PowerPoint Presentation, free Why Do Catalysts Lower The Activation Energy Find out how temperature affects the activation energy and the. learn how activation energy is the energy required for a reaction to occur and determines its rate. — activation energy is the minimum energy needed to start a chemical reaction. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. catalysts. Why Do Catalysts Lower The Activation Energy.
From schematicbraginamh.z4.web.core.windows.net
Energy Diagram For Chemical Reaction Why Do Catalysts Lower The Activation Energy catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. See examples, diagrams and explanations of the. Find out how temperature affects the activation energy and the. — activation energy is the minimum energy needed to start a chemical reaction. learn the fundamental principles of catalysis and how. Why Do Catalysts Lower The Activation Energy.
From www.slideserve.com
PPT Chapter 5 Enzymes PowerPoint Presentation, free download ID Why Do Catalysts Lower The Activation Energy — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. learn how activation energy is the energy required for a reaction to occur and determines its rate. Find out how temperature affects the activation energy and the. — activation energy is the minimum energy needed to start a. Why Do Catalysts Lower The Activation Energy.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Why Do Catalysts Lower The Activation Energy learn how catalysts speed up reactions by providing an alternative route with lower activation energy. See examples, diagrams and explanations of the. Learn how to calculate it, how it affects reaction rate, and. — activation energy is the minimum energy needed to start a chemical reaction. — learn how catalysts speed up reactions by lowering the activation. Why Do Catalysts Lower The Activation Energy.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Why Do Catalysts Lower The Activation Energy See examples, diagrams and explanations of the. learn the fundamental principles of catalysis and how the mechanisms are studied with examples and diagrams. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Learn. Why Do Catalysts Lower The Activation Energy.
From 2012books.lardbucket.org
Catalysis Why Do Catalysts Lower The Activation Energy Find out how temperature affects the activation energy and the. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. learn the fundamental principles of catalysis and how the mechanisms are studied with examples and diagrams. Learn how to calculate it, how it affects reaction rate, and. catalysts make. Why Do Catalysts Lower The Activation Energy.
From www.slideserve.com
PPT Enzymes PowerPoint Presentation, free download ID1936659 Why Do Catalysts Lower The Activation Energy learn the fundamental principles of catalysis and how the mechanisms are studied with examples and diagrams. See examples, diagrams and explanations of the. — activation energy is the minimum energy needed to start a chemical reaction. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. learn. Why Do Catalysts Lower The Activation Energy.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Why Do Catalysts Lower The Activation Energy Learn how to calculate it, how it affects reaction rate, and. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. — catalysts are defined as substances that participate in a chemical reaction. Why Do Catalysts Lower The Activation Energy.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology Why Do Catalysts Lower The Activation Energy learn how catalysts speed up reactions by providing an alternative route with lower activation energy. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. See examples, diagrams and explanations of the. — activation energy is the minimum energy needed to start a chemical reaction. catalysts make this. Why Do Catalysts Lower The Activation Energy.
From exoajjasl.blob.core.windows.net
A Catalyst Lowers The Activation Energy Of A Reaction From 20 To 10 at Why Do Catalysts Lower The Activation Energy — activation energy is the minimum energy needed to start a chemical reaction. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. See examples, diagrams and explanations of the. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. — catalysts. Why Do Catalysts Lower The Activation Energy.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Why Do Catalysts Lower The Activation Energy Find out how temperature affects the activation energy and the. — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. See examples, diagrams and explanations of the. learn how activation energy is the. Why Do Catalysts Lower The Activation Energy.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Why Do Catalysts Lower The Activation Energy learn how catalysts speed up reactions by providing an alternative route with lower activation energy. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. — activation energy is. Why Do Catalysts Lower The Activation Energy.
From www.chim.lu
Activation energy and catalysis Why Do Catalysts Lower The Activation Energy learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how activation energy is the energy required for a reaction to occur and determines its rate. Find out how temperature affects the activation energy and the. — catalysts are defined as substances that participate in a chemical reaction but are not. Why Do Catalysts Lower The Activation Energy.
From www.slideserve.com
PPT Reaction PowerPoint Presentation ID1835084 Why Do Catalysts Lower The Activation Energy — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. — activation energy is the minimum energy needed to start a chemical reaction. See examples, diagrams and explanations of the.. Why Do Catalysts Lower The Activation Energy.
From www.onlinebiologynotes.com
Enzymes Properties and Mechanism of enzyme action Online Biology Notes Why Do Catalysts Lower The Activation Energy Learn how to calculate it, how it affects reaction rate, and. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. — activation energy is the minimum energy needed to start a chemical reaction. learn how catalysts speed up reactions by providing an alternative route with lower activation energy.. Why Do Catalysts Lower The Activation Energy.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Why Do Catalysts Lower The Activation Energy Learn how to calculate it, how it affects reaction rate, and. — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. See examples, diagrams and explanations of the. catalysts make. Why Do Catalysts Lower The Activation Energy.
From www.slideserve.com
PPT Enzymes Biological Catalysts PowerPoint Presentation ID5736986 Why Do Catalysts Lower The Activation Energy catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. See examples, diagrams and explanations of the. learn how activation energy is the energy required for a reaction to occur and determines its rate. Learn how to calculate it, how it affects reaction rate, and. — activation energy. Why Do Catalysts Lower The Activation Energy.
From dxobwughx.blob.core.windows.net
Do All Enzymes Lower Activation Energy at John Lucchesi blog Why Do Catalysts Lower The Activation Energy Learn how to calculate it, how it affects reaction rate, and. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. — catalysts are defined as substances that participate in a chemical reaction. Why Do Catalysts Lower The Activation Energy.
From slideplayer.com
Chemical Reactions. ppt download Why Do Catalysts Lower The Activation Energy learn the fundamental principles of catalysis and how the mechanisms are studied with examples and diagrams. Find out how temperature affects the activation energy and the. — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. Learn how to calculate it, how it affects reaction rate, and. learn. Why Do Catalysts Lower The Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Why Do Catalysts Lower The Activation Energy — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. See examples, diagrams and explanations of the. Learn how to calculate it, how it affects reaction rate, and. learn how. Why Do Catalysts Lower The Activation Energy.
From byjus.com
A catalyst lowers the activation energy of the forward reaction by 20 Why Do Catalysts Lower The Activation Energy — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. learn how activation energy is the energy required for a reaction to occur and determines its rate. Find out how temperature affects the activation energy and the. learn the fundamental principles of catalysis and how the mechanisms are. Why Do Catalysts Lower The Activation Energy.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Why Do Catalysts Lower The Activation Energy Find out how temperature affects the activation energy and the. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how activation energy is the energy required for a reaction to occur and determines its rate. See examples, diagrams and explanations of the. learn the fundamental principles of catalysis and how. Why Do Catalysts Lower The Activation Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Why Do Catalysts Lower The Activation Energy — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. learn the fundamental principles of catalysis and how the mechanisms are studied with examples and diagrams. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. — activation energy is the minimum. Why Do Catalysts Lower The Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Why Do Catalysts Lower The Activation Energy Find out how temperature affects the activation energy and the. — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. See examples, diagrams and explanations of the. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. Learn how to calculate. Why Do Catalysts Lower The Activation Energy.
From www.sliderbase.com
Catalysis Presentation Chemistry Why Do Catalysts Lower The Activation Energy Learn how to calculate it, how it affects reaction rate, and. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. learn the fundamental principles of catalysis and how the mechanisms are studied with examples and diagrams. learn how catalysts speed up reactions by providing an alternative route with. Why Do Catalysts Lower The Activation Energy.
From www.slideserve.com
PPT Metabolism PowerPoint Presentation, free download ID1153506 Why Do Catalysts Lower The Activation Energy Find out how temperature affects the activation energy and the. — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. learn how activation energy is the energy required for a reaction to occur and determines its rate. Learn how to calculate it, how it affects reaction rate, and. . Why Do Catalysts Lower The Activation Energy.
From studylib.net
Enzymes Lower Activation Energy Why Do Catalysts Lower The Activation Energy — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. Find out how temperature affects the activation energy and the. learn the fundamental principles of catalysis and how the mechanisms are studied with examples and diagrams. learn how catalysts speed up reactions by providing an alternative route with. Why Do Catalysts Lower The Activation Energy.
From exohcytva.blob.core.windows.net
How Do Enzymes Lower Activation Energy Quizlet at Su Happel blog Why Do Catalysts Lower The Activation Energy — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. Find out how temperature affects the activation energy and the. Learn how to calculate it, how it affects reaction rate,. Why Do Catalysts Lower The Activation Energy.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Why Do Catalysts Lower The Activation Energy learn how catalysts speed up reactions by providing an alternative route with lower activation energy. — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. learn the fundamental principles. Why Do Catalysts Lower The Activation Energy.
From www.slideserve.com
PPT Enzyme and Catalysis PowerPoint Presentation, free Why Do Catalysts Lower The Activation Energy learn the fundamental principles of catalysis and how the mechanisms are studied with examples and diagrams. learn how catalysts speed up reactions by providing an alternative route with lower activation energy. learn how activation energy is the energy required for a reaction to occur and determines its rate. — activation energy is the minimum energy needed. Why Do Catalysts Lower The Activation Energy.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Why Do Catalysts Lower The Activation Energy — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. Find out how temperature affects the activation energy and the. catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. — activation energy is the minimum energy needed to. Why Do Catalysts Lower The Activation Energy.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Why Do Catalysts Lower The Activation Energy catalysts make this process more efficient by lowering the activation energy, which is the energy barrier that must be. learn the fundamental principles of catalysis and how the mechanisms are studied with examples and diagrams. — activation energy is the minimum energy needed to start a chemical reaction. learn how activation energy is the energy required. Why Do Catalysts Lower The Activation Energy.
From slideplayer.com
Enzymes. ppt download Why Do Catalysts Lower The Activation Energy learn how catalysts speed up reactions by providing an alternative route with lower activation energy. — learn how catalysts speed up reactions by lowering the activation energy and increasing the number of successful collisions. — catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalysts make this process. Why Do Catalysts Lower The Activation Energy.