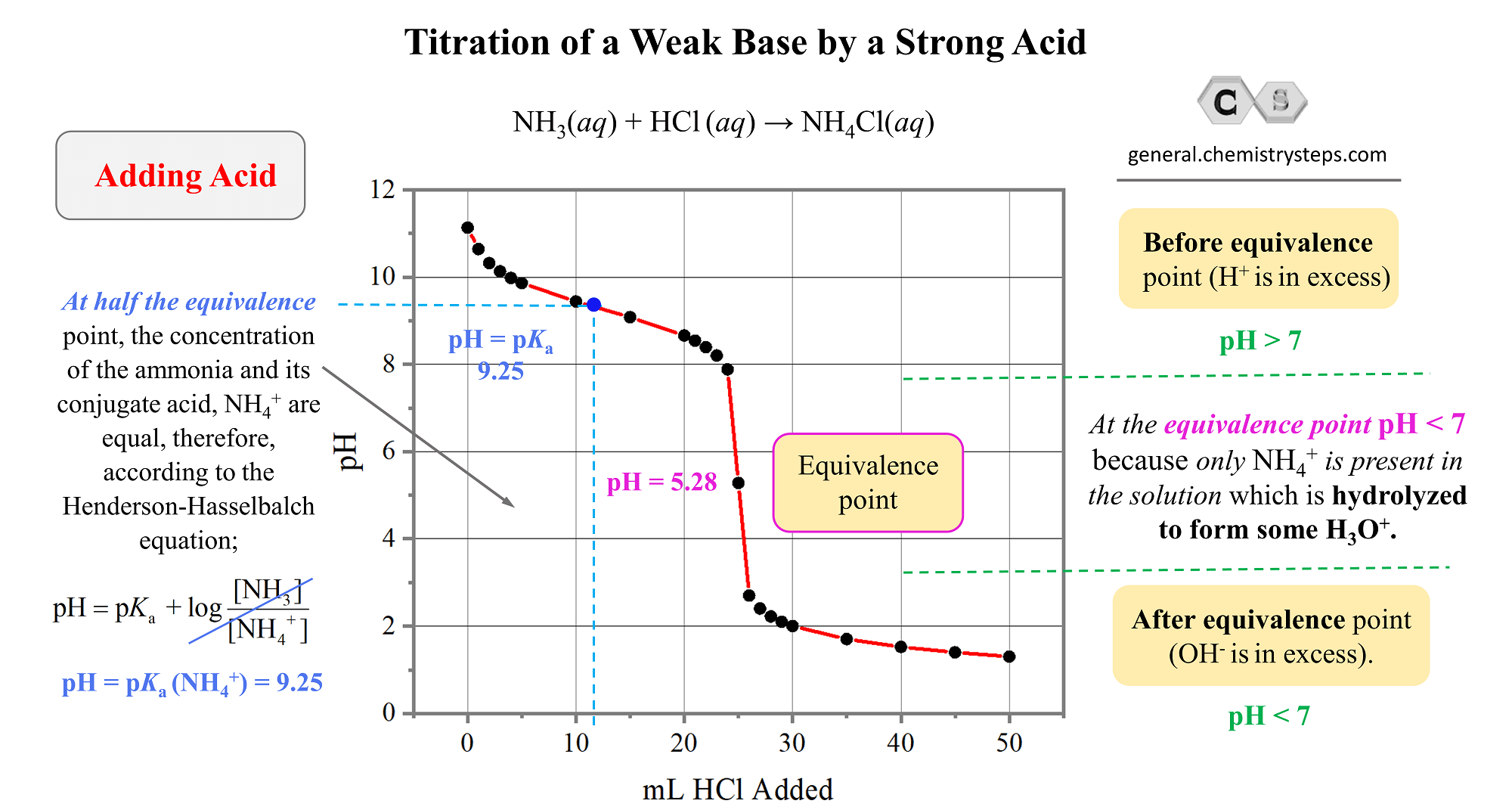
Buffer And Titration Questions . What i absolutely have to know to survive the ap exam. Consider the titration of 25.00 ml of 0.1000 m. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? What is the ph of this buffer solution? A plot of ph versus volume of base added gives what is known as a titration curve. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The following might indicate the question deals with buffers and/or titrations:
from ar.inspiredpencil.com
Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? The following might indicate the question deals with buffers and/or titrations: In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. What is the ph of this buffer solution? What i absolutely have to know to survive the ap exam. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Consider the titration of 25.00 ml of 0.1000 m. A plot of ph versus volume of base added gives what is known as a titration curve.
Diagram Of Acid Base Titration
Buffer And Titration Questions A plot of ph versus volume of base added gives what is known as a titration curve. What i absolutely have to know to survive the ap exam. Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? Consider the titration of 25.00 ml of 0.1000 m. What is the ph of this buffer solution? In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. A plot of ph versus volume of base added gives what is known as a titration curve. The following might indicate the question deals with buffers and/or titrations: Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution).
From www.studocu.com
217 buffer, titration problems CHEM 217 Studocu Buffer And Titration Questions A plot of ph versus volume of base added gives what is known as a titration curve. Consider the titration of 25.00 ml of 0.1000 m. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Which buffer component must be added,. Buffer And Titration Questions.
From edzion.com
HSC Titration Questions Edzion Buffer And Titration Questions Consider the titration of 25.00 ml of 0.1000 m. What is the ph of this buffer solution? Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). The following might indicate the question deals with buffers and/or titrations: In this section, we will see how to perform calculations to predict. Buffer And Titration Questions.
From mavink.com
Titration Labeled Buffer And Titration Questions Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Consider the titration of 25.00 ml of 0.1000 m. What is the ph of this buffer solution? In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak. Buffer And Titration Questions.
From www.chegg.com
Solved Part 2 Titration of Acetate Buffer with HCl and NaOH Buffer And Titration Questions The following might indicate the question deals with buffers and/or titrations: Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? What is the ph of this buffer solution? A plot of ph versus volume of base added gives what is known as a titration curve. Consider the titration of 25.00 ml of. Buffer And Titration Questions.
From exovevmgr.blob.core.windows.net
Titration Lab Quiz at Roger Owen blog Buffer And Titration Questions Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). What is the ph of this buffer solution? In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Which buffer component must. Buffer And Titration Questions.
From www.coursehero.com
[Solved] ii. Below is a titration curve for carbonic acid. Indicate key Buffer And Titration Questions Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak. Buffer And Titration Questions.
From dxorulukz.blob.core.windows.net
Titration Practice Questions A Level at Hiles blog Buffer And Titration Questions What i absolutely have to know to survive the ap exam. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? The following might indicate. Buffer And Titration Questions.
From exopchdlm.blob.core.windows.net
Titration Curve Quiz at Miranda blog Buffer And Titration Questions Consider the titration of 25.00 ml of 0.1000 m. Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? What is the ph of this buffer solution? The following might indicate the question deals with buffers and/or titrations: A plot of ph versus volume of base added gives what is known as a. Buffer And Titration Questions.
From socratic.org
Is a solution of a weak acid considered a buffer solution? Socratic Buffer And Titration Questions Consider the titration of 25.00 ml of 0.1000 m. What i absolutely have to know to survive the ap exam. Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak. Buffer And Titration Questions.
From exyouyqrf.blob.core.windows.net
Titration Apparatus And Uses at Nicole Fain blog Buffer And Titration Questions What i absolutely have to know to survive the ap exam. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). A plot of ph versus volume of base added gives what is known as a titration curve. The following might indicate the question deals with buffers and/or titrations: What. Buffer And Titration Questions.
From alkermesxkpmaterialdb.z13.web.core.windows.net
Chemistry Titration Questions And Answers Buffer And Titration Questions Consider the titration of 25.00 ml of 0.1000 m. A plot of ph versus volume of base added gives what is known as a titration curve. What is the ph of this buffer solution? In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base,. Buffer And Titration Questions.
From www.studocu.com
Lecture 5 Exp 4 Buffer Solutions and Titration Curves Lecture 5 Exp Buffer And Titration Questions A plot of ph versus volume of base added gives what is known as a titration curve. Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base,. Buffer And Titration Questions.
From klaqnshvp.blob.core.windows.net
How To Do A Titration Rsc at Paul Gallegos blog Buffer And Titration Questions Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Consider the titration of 25.00 ml of 0.1000 m. The following might indicate the question deals with buffers and/or titrations: Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? In this section,. Buffer And Titration Questions.
From joitysbjy.blob.core.windows.net
Edexcel Titration Questions at Threadgill blog Buffer And Titration Questions In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. What i absolutely have to know to survive the ap exam. A plot of ph versus volume of base added gives what is known as a titration curve. Which buffer component must. Buffer And Titration Questions.
From www.chegg.com
Solved Consider the following questions about buffer Buffer And Titration Questions Consider the titration of 25.00 ml of 0.1000 m. What is the ph of this buffer solution? Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). The following might indicate the question. Buffer And Titration Questions.
From www.youtube.com
Acids and Bases Buffer Calculation Past Paper Exam Question Buffer And Titration Questions In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. What i absolutely have to know to survive the ap exam. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). What. Buffer And Titration Questions.
From ar.inspiredpencil.com
Diagram Of Acid Base Titration Buffer And Titration Questions A plot of ph versus volume of base added gives what is known as a titration curve. The following might indicate the question deals with buffers and/or titrations: What i absolutely have to know to survive the ap exam. In this section, we will see how to perform calculations to predict the ph at any point in a titration of. Buffer And Titration Questions.
From www.studypool.com
SOLUTION Igcse chem topic questions titration Studypool Buffer And Titration Questions What is the ph of this buffer solution? Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Consider the titration of 25.00 ml of 0.1000 m. What i absolutely have to know to survive the ap exam. A plot of ph versus volume of base added gives what is. Buffer And Titration Questions.
From joieldmit.blob.core.windows.net
How Is Titration Used In Medicine at Shelia Wiley blog Buffer And Titration Questions A plot of ph versus volume of base added gives what is known as a titration curve. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? What i absolutely have to know. Buffer And Titration Questions.
From exyobmsds.blob.core.windows.net
AcidBase Titration Lab Advanced Chemistry With Vernier Answers at Buffer And Titration Questions In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. The following might indicate the question deals with buffers and/or titrations: Consider the titration of 25.00 ml of 0.1000 m. A plot of ph versus volume of base added gives what is. Buffer And Titration Questions.
From www.madebyteachers.com
Solution and Titration Stoichiometry a Chemistry Worksheet Made By Buffer And Titration Questions What i absolutely have to know to survive the ap exam. The following might indicate the question deals with buffers and/or titrations: A plot of ph versus volume of base added gives what is known as a titration curve. In this section, we will see how to perform calculations to predict the ph at any point in a titration of. Buffer And Titration Questions.
From mmerevise.co.uk
pH Curves Questions and Revision MME Buffer And Titration Questions In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. A plot of ph versus volume of base added gives what is known as a titration curve. Which buffer component must be added, and in what quantity, to change the buffer ph. Buffer And Titration Questions.
From mavink.com
Buffer Region Titration Curve Buffer And Titration Questions What is the ph of this buffer solution? What i absolutely have to know to survive the ap exam. Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? A plot of ph versus volume of base added gives what is known as a titration curve. Consider the titration of 25.00 ml of. Buffer And Titration Questions.
From joitysbjy.blob.core.windows.net
Edexcel Titration Questions at Threadgill blog Buffer And Titration Questions What i absolutely have to know to survive the ap exam. The following might indicate the question deals with buffers and/or titrations: Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? What is the ph of this buffer solution? A plot of ph versus volume of base added gives what is known. Buffer And Titration Questions.
From www.studypool.com
SOLUTION Igcse chem topic questions titration Studypool Buffer And Titration Questions A plot of ph versus volume of base added gives what is known as a titration curve. Consider the titration of 25.00 ml of 0.1000 m. What is the ph of this buffer solution? Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). The following might indicate the question. Buffer And Titration Questions.
From mavink.com
Phosphate Titration Curve Buffer And Titration Questions In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. What is the ph of this buffer solution? Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? Consider the titration of 25.00 ml of. Buffer And Titration Questions.
From www.studypool.com
SOLUTION Igcse chem topic questions titration Studypool Buffer And Titration Questions What i absolutely have to know to survive the ap exam. In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. A plot of ph versus volume of base added gives what is known as a titration curve. Consider the titration of. Buffer And Titration Questions.
From www.youtube.com
GCII Buffers and Titrations practice problems. YouTube Buffer And Titration Questions Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Consider the titration of 25.00 ml of 0.1000 m. The following might indicate the question. Buffer And Titration Questions.
From klajqxgeq.blob.core.windows.net
Titration Question Examples at Larry Magno blog Buffer And Titration Questions The following might indicate the question deals with buffers and/or titrations: A plot of ph versus volume of base added gives what is known as a titration curve. What is the ph of this buffer solution? Consider the titration of 25.00 ml of 0.1000 m. What i absolutely have to know to survive the ap exam. Which buffer component must. Buffer And Titration Questions.
From www.youtube.com
Titration Curves for High School Chemistry YouTube Buffer And Titration Questions A plot of ph versus volume of base added gives what is known as a titration curve. The following might indicate the question deals with buffers and/or titrations: What is the ph of this buffer solution? Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? In this section, we will see how. Buffer And Titration Questions.
From kaytrinasofya.blogspot.com
37+ titration calculation questions gcse Buffer And Titration Questions Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). Consider the titration of 25.00 ml of 0.1000 m. A plot of ph versus volume of base added gives what is known as a titration curve. The following might indicate the question deals with buffers and/or titrations: What is the. Buffer And Titration Questions.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE Buffer And Titration Questions Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. What i absolutely have to know to survive the ap exam. The following might indicate. Buffer And Titration Questions.
From www.bartleby.com
Answered At what point on the titration curve… bartleby Buffer And Titration Questions What i absolutely have to know to survive the ap exam. The following might indicate the question deals with buffers and/or titrations: In this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using the. Which buffer component must be added, and in what quantity,. Buffer And Titration Questions.
From www.studypool.com
SOLUTION Titrations questions with answers Studypool Buffer And Titration Questions Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? A plot of ph versus volume of base added gives what is known as a titration curve. Explain how a buffer solution manages to stabilize the ph against the addition of acid, base, or additional solvent (dilution). In this section, we will see. Buffer And Titration Questions.
From www.coursehero.com
[Solved] . D Question 23 3 pts Please match the following titration Buffer And Titration Questions Which buffer component must be added, and in what quantity, to change the buffer ph to 4.00? What is the ph of this buffer solution? A plot of ph versus volume of base added gives what is known as a titration curve. In this section, we will see how to perform calculations to predict the ph at any point in. Buffer And Titration Questions.