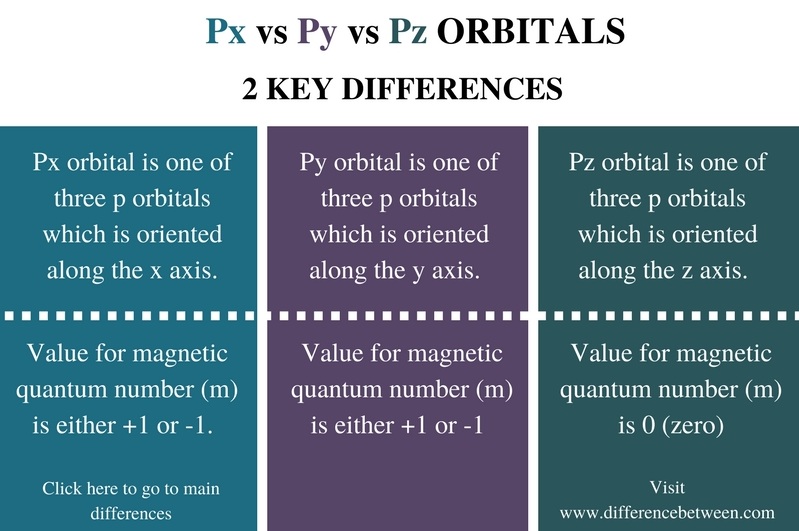
What Is Px Py Pz In Chemistry . writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and. The x, y, and z directions change. these are arbitrarily given the symbols px, py and pz. However, there are some key differences. This is simply for convenience; px, py, pz → 3 fold degenerate. D orbital shapes → 5 fold degenerate. px and py orbitals are both part of the set of three p orbitals, along with the pz orbital. Revision notes on 1.1.4 shells and orbitals for the. in ions with only a single electron, the energy of a given orbital depends on only n, and all subshells within a principal shell, such as. our chemistry teacher said that two pz p z orbital only form sigma bond whereas two px p x and two py p y orbitals form only pi bond. An atomic orbital is a.
from www.differencebetween.com
px and py orbitals are both part of the set of three p orbitals, along with the pz orbital. our chemistry teacher said that two pz p z orbital only form sigma bond whereas two px p x and two py p y orbitals form only pi bond. This is simply for convenience; D orbital shapes → 5 fold degenerate. An atomic orbital is a. The x, y, and z directions change. these are arbitrarily given the symbols px, py and pz. writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and. Revision notes on 1.1.4 shells and orbitals for the. However, there are some key differences.
Difference Between Px Py and Pz Orbitals Compare the Difference
What Is Px Py Pz In Chemistry Revision notes on 1.1.4 shells and orbitals for the. these are arbitrarily given the symbols px, py and pz. px, py, pz → 3 fold degenerate. This is simply for convenience; px and py orbitals are both part of the set of three p orbitals, along with the pz orbital. Revision notes on 1.1.4 shells and orbitals for the. However, there are some key differences. our chemistry teacher said that two pz p z orbital only form sigma bond whereas two px p x and two py p y orbitals form only pi bond. writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and. An atomic orbital is a. D orbital shapes → 5 fold degenerate. in ions with only a single electron, the energy of a given orbital depends on only n, and all subshells within a principal shell, such as. The x, y, and z directions change.
From www.shutterstock.com
P Atomic Orbital Pxpy Pz Stock Illustration 1639712359 Shutterstock What Is Px Py Pz In Chemistry However, there are some key differences. our chemistry teacher said that two pz p z orbital only form sigma bond whereas two px p x and two py p y orbitals form only pi bond. An atomic orbital is a. these are arbitrarily given the symbols px, py and pz. This is simply for convenience; Revision notes on. What Is Px Py Pz In Chemistry.
From thenoveldifference.com
Px Py and Pz Orbitals The best 5 difference What Is Px Py Pz In Chemistry px and py orbitals are both part of the set of three p orbitals, along with the pz orbital. However, there are some key differences. Revision notes on 1.1.4 shells and orbitals for the. An atomic orbital is a. This is simply for convenience; writing out px, py, pz fully or using p^n notation (n=no of electrons in. What Is Px Py Pz In Chemistry.
From chemistryteory.blogspot.com
chemistry Linear Combination of Atomic Orbitals px, py and pz What Is Px Py Pz In Chemistry Revision notes on 1.1.4 shells and orbitals for the. in ions with only a single electron, the energy of a given orbital depends on only n, and all subshells within a principal shell, such as. writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and.. What Is Px Py Pz In Chemistry.
From www.researchgate.net
Atom orbital projection of s, p x , p y and p z orbitals for the atoms What Is Px Py Pz In Chemistry Revision notes on 1.1.4 shells and orbitals for the. An atomic orbital is a. px, py, pz → 3 fold degenerate. writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and. This is simply for convenience; our chemistry teacher said that two pz p. What Is Px Py Pz In Chemistry.
From www.youtube.com
12.1.5 Draw the shape of an s orbital and the shapes of the p x , p y What Is Px Py Pz In Chemistry writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and. these are arbitrarily given the symbols px, py and pz. The x, y, and z directions change. D orbital shapes → 5 fold degenerate. px and py orbitals are both part of the set. What Is Px Py Pz In Chemistry.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent What Is Px Py Pz In Chemistry px and py orbitals are both part of the set of three p orbitals, along with the pz orbital. writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and. The x, y, and z directions change. This is simply for convenience; An atomic orbital is. What Is Px Py Pz In Chemistry.
From www.askiitians.com
Draw the atomic orbital overlaps pxpx pxpy pzpz pzpy pzs under w What Is Px Py Pz In Chemistry these are arbitrarily given the symbols px, py and pz. However, there are some key differences. D orbital shapes → 5 fold degenerate. This is simply for convenience; Revision notes on 1.1.4 shells and orbitals for the. px and py orbitals are both part of the set of three p orbitals, along with the pz orbital. The x,. What Is Px Py Pz In Chemistry.
From www.masterorganicchemistry.com
Review of Atomic Orbitals Master Organic Chemistry What Is Px Py Pz In Chemistry px, py, pz → 3 fold degenerate. D orbital shapes → 5 fold degenerate. writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and. This is simply for convenience; px and py orbitals are both part of the set of three p orbitals, along. What Is Px Py Pz In Chemistry.
From www.researchgate.net
Combinations of chalcogen pz (a) and px, pyorbitals (b) according to What Is Px Py Pz In Chemistry Revision notes on 1.1.4 shells and orbitals for the. px and py orbitals are both part of the set of three p orbitals, along with the pz orbital. px, py, pz → 3 fold degenerate. An atomic orbital is a. However, there are some key differences. This is simply for convenience; The x, y, and z directions change.. What Is Px Py Pz In Chemistry.
From www.chegg.com
Solved Classify these atomic orbital as px Py, or pz. px Py What Is Px Py Pz In Chemistry This is simply for convenience; px and py orbitals are both part of the set of three p orbitals, along with the pz orbital. these are arbitrarily given the symbols px, py and pz. D orbital shapes → 5 fold degenerate. px, py, pz → 3 fold degenerate. An atomic orbital is a. in ions with. What Is Px Py Pz In Chemistry.
From www.reddit.com
Why do Px and Py orbitals form Pi bonds while Pz forms a sigma mo What Is Px Py Pz In Chemistry D orbital shapes → 5 fold degenerate. The x, y, and z directions change. Revision notes on 1.1.4 shells and orbitals for the. px and py orbitals are both part of the set of three p orbitals, along with the pz orbital. these are arbitrarily given the symbols px, py and pz. writing out px, py, pz. What Is Px Py Pz In Chemistry.
From www.slideserve.com
PPT Chapter 1 Structure and Bonding PowerPoint Presentation, free What Is Px Py Pz In Chemistry An atomic orbital is a. px, py, pz → 3 fold degenerate. Revision notes on 1.1.4 shells and orbitals for the. However, there are some key differences. This is simply for convenience; these are arbitrarily given the symbols px, py and pz. px and py orbitals are both part of the set of three p orbitals, along. What Is Px Py Pz In Chemistry.
From www.chemistrynotmystery.com
Linear Combination of Atomic Orbitals px, py and pz Chemistry!!! Not What Is Px Py Pz In Chemistry However, there are some key differences. Revision notes on 1.1.4 shells and orbitals for the. in ions with only a single electron, the energy of a given orbital depends on only n, and all subshells within a principal shell, such as. our chemistry teacher said that two pz p z orbital only form sigma bond whereas two px. What Is Px Py Pz In Chemistry.
From byjus.com
Name the type of bond formed by 1 Px+Px orbital on y or z What Is Px Py Pz In Chemistry our chemistry teacher said that two pz p z orbital only form sigma bond whereas two px p x and two py p y orbitals form only pi bond. writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and. An atomic orbital is a. . What Is Px Py Pz In Chemistry.
From www.pinclipart.com
The Px, Py, And Pz Atomic Orbitals Of Carbon Atomic Orbital Clipart What Is Px Py Pz In Chemistry This is simply for convenience; our chemistry teacher said that two pz p z orbital only form sigma bond whereas two px p x and two py p y orbitals form only pi bond. D orbital shapes → 5 fold degenerate. Revision notes on 1.1.4 shells and orbitals for the. The x, y, and z directions change. px. What Is Px Py Pz In Chemistry.
From www.numerade.com
SOLVED Identify the orbital pz orbital dxy orbital px orbital dyz What Is Px Py Pz In Chemistry these are arbitrarily given the symbols px, py and pz. However, there are some key differences. writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and. in ions with only a single electron, the energy of a given orbital depends on only n, and. What Is Px Py Pz In Chemistry.
From slidetodoc.com
Structure and Bonding in Organic Chemistry Electron Configurations What Is Px Py Pz In Chemistry However, there are some key differences. our chemistry teacher said that two pz p z orbital only form sigma bond whereas two px p x and two py p y orbitals form only pi bond. Revision notes on 1.1.4 shells and orbitals for the. D orbital shapes → 5 fold degenerate. The x, y, and z directions change. . What Is Px Py Pz In Chemistry.
From www.youtube.com
Shape of atomic orbitals ( px,py,pz) detailed lecture chemistry xi What Is Px Py Pz In Chemistry However, there are some key differences. these are arbitrarily given the symbols px, py and pz. our chemistry teacher said that two pz p z orbital only form sigma bond whereas two px p x and two py p y orbitals form only pi bond. An atomic orbital is a. px and py orbitals are both part. What Is Px Py Pz In Chemistry.
From www.chegg.com
12. Consider the three porbitals (px, py and pz What Is Px Py Pz In Chemistry our chemistry teacher said that two pz p z orbital only form sigma bond whereas two px p x and two py p y orbitals form only pi bond. px, py, pz → 3 fold degenerate. writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of. What Is Px Py Pz In Chemistry.
From www.chemistrynotmystery.com
Linear Combination of Atomic Orbitals px, py and pz Chemistry!!! Not What Is Px Py Pz In Chemistry The x, y, and z directions change. in ions with only a single electron, the energy of a given orbital depends on only n, and all subshells within a principal shell, such as. these are arbitrarily given the symbols px, py and pz. An atomic orbital is a. our chemistry teacher said that two pz p z. What Is Px Py Pz In Chemistry.
From chemistryskills.com
Shapes of Orbitals and their Types Chemistry Skills What Is Px Py Pz In Chemistry However, there are some key differences. D orbital shapes → 5 fold degenerate. The x, y, and z directions change. our chemistry teacher said that two pz p z orbital only form sigma bond whereas two px p x and two py p y orbitals form only pi bond. px, py, pz → 3 fold degenerate. Revision notes. What Is Px Py Pz In Chemistry.
From chemistryteory.blogspot.com
chemistry Linear Combination of Atomic Orbitals px, py and pz What Is Px Py Pz In Chemistry px and py orbitals are both part of the set of three p orbitals, along with the pz orbital. writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and. our chemistry teacher said that two pz p z orbital only form sigma bond whereas. What Is Px Py Pz In Chemistry.
From www.pinclipart.com
The Px, Py, And Pz Atomic Orbitals Of Carbon Atomic Orbital Clipart What Is Px Py Pz In Chemistry writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and. px and py orbitals are both part of the set of three p orbitals, along with the pz orbital. The x, y, and z directions change. these are arbitrarily given the symbols px, py. What Is Px Py Pz In Chemistry.
From www.youtube.com
Atomicorbital 3D 3D Atomic orbitals Px,Py,Pz 3D representation What Is Px Py Pz In Chemistry Revision notes on 1.1.4 shells and orbitals for the. these are arbitrarily given the symbols px, py and pz. An atomic orbital is a. px and py orbitals are both part of the set of three p orbitals, along with the pz orbital. The x, y, and z directions change. However, there are some key differences. D orbital. What Is Px Py Pz In Chemistry.
From www.differencebetween.com
Difference Between Px Py and Pz Orbitals Compare the Difference What Is Px Py Pz In Chemistry px, py, pz → 3 fold degenerate. writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and. The x, y, and z directions change. our chemistry teacher said that two pz p z orbital only form sigma bond whereas two px p x and. What Is Px Py Pz In Chemistry.
From www.researchgate.net
(a) A tightbinding model on a simple cubic lattice with s, px, py, and What Is Px Py Pz In Chemistry An atomic orbital is a. This is simply for convenience; in ions with only a single electron, the energy of a given orbital depends on only n, and all subshells within a principal shell, such as. px, py, pz → 3 fold degenerate. The x, y, and z directions change. However, there are some key differences. D orbital. What Is Px Py Pz In Chemistry.
From www.researchgate.net
Orbitalprojected band structures of px, py, pz orbitals of Sb and Bi What Is Px Py Pz In Chemistry This is simply for convenience; Revision notes on 1.1.4 shells and orbitals for the. these are arbitrarily given the symbols px, py and pz. px, py, pz → 3 fold degenerate. An atomic orbital is a. writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of. What Is Px Py Pz In Chemistry.
From www.meritnation.com
Why mot structurd of N2 is different from O2 in comparison when we see What Is Px Py Pz In Chemistry these are arbitrarily given the symbols px, py and pz. This is simply for convenience; D orbital shapes → 5 fold degenerate. The x, y, and z directions change. An atomic orbital is a. in ions with only a single electron, the energy of a given orbital depends on only n, and all subshells within a principal shell,. What Is Px Py Pz In Chemistry.
From www.researchgate.net
Polarizations Px, Py, and Pz during heatingup and coolingdown What Is Px Py Pz In Chemistry px, py, pz → 3 fold degenerate. these are arbitrarily given the symbols px, py and pz. in ions with only a single electron, the energy of a given orbital depends on only n, and all subshells within a principal shell, such as. writing out px, py, pz fully or using p^n notation (n=no of electrons. What Is Px Py Pz In Chemistry.
From www.researchgate.net
The projection of the total band structure onto the {s,px,py,pz What Is Px Py Pz In Chemistry D orbital shapes → 5 fold degenerate. However, there are some key differences. px, py, pz → 3 fold degenerate. in ions with only a single electron, the energy of a given orbital depends on only n, and all subshells within a principal shell, such as. This is simply for convenience; px and py orbitals are both. What Is Px Py Pz In Chemistry.
From www.bartleby.com
Answered y One s orbital Px Py Three p orbitals… bartleby What Is Px Py Pz In Chemistry However, there are some key differences. The x, y, and z directions change. our chemistry teacher said that two pz p z orbital only form sigma bond whereas two px p x and two py p y orbitals form only pi bond. px, py, pz → 3 fold degenerate. in ions with only a single electron, the. What Is Px Py Pz In Chemistry.
From www.technocrazed.com
Orbitals (s) Three fold symmetry. (p) Shown px, one of three possible What Is Px Py Pz In Chemistry Revision notes on 1.1.4 shells and orbitals for the. This is simply for convenience; in ions with only a single electron, the energy of a given orbital depends on only n, and all subshells within a principal shell, such as. However, there are some key differences. px and py orbitals are both part of the set of three. What Is Px Py Pz In Chemistry.
From www.numerade.com
SOLVED Identify the orbital y pz orbital dxy orbital px orbital dyz What Is Px Py Pz In Chemistry writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and. An atomic orbital is a. The x, y, and z directions change. D orbital shapes → 5 fold degenerate. these are arbitrarily given the symbols px, py and pz. However, there are some key differences.. What Is Px Py Pz In Chemistry.
From www.youtube.com
12.1.5 (New Syl 2.3) Draw the shape of an s orbital px, py and pz What Is Px Py Pz In Chemistry px, py, pz → 3 fold degenerate. these are arbitrarily given the symbols px, py and pz. px and py orbitals are both part of the set of three p orbitals, along with the pz orbital. Revision notes on 1.1.4 shells and orbitals for the. our chemistry teacher said that two pz p z orbital only. What Is Px Py Pz In Chemistry.
From www.differencebetween.com
Difference Between Px Py and Pz Orbitals Compare the Difference What Is Px Py Pz In Chemistry these are arbitrarily given the symbols px, py and pz. This is simply for convenience; writing out px, py, pz fully or using p^n notation (n=no of electrons in a p orbital) is a matter of context and. Revision notes on 1.1.4 shells and orbitals for the. However, there are some key differences. px, py, pz →. What Is Px Py Pz In Chemistry.