What Is A Buffer Medical Term . The term buffer was introduced to describe the ability of a solution to tolerate ph changes when exposed to strong acids or bases, allowing almost all physiological processes in the body to occur. A buffer typically consists of a. The four examples of physiological buffers are here. Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. A solution used to stabilize the ph. Buffers are substances that help maintain the ph of a solution within a specific range. Buffers are solutions that contain a weak acid and its a conjugate base; A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions consist of a weak acid and its. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph.
from loexxrihd.blob.core.windows.net
Buffers are solutions that contain a weak acid and its a conjugate base; A solution used to stabilize the ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. The term buffer was introduced to describe the ability of a solution to tolerate ph changes when exposed to strong acids or bases, allowing almost all physiological processes in the body to occur. Buffer solutions consist of a weak acid and its. A buffer typically consists of a. The four examples of physiological buffers are here. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. Buffers are substances that help maintain the ph of a solution within a specific range.
What Is The Function Of A Buffer What Is A Buffer Made From at
What Is A Buffer Medical Term The four examples of physiological buffers are here. A buffer typically consists of a. A solution used to stabilize the ph. Buffers are solutions that contain a weak acid and its a conjugate base; Buffers are substances that help maintain the ph of a solution within a specific range. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. The four examples of physiological buffers are here. Buffer solutions consist of a weak acid and its. The term buffer was introduced to describe the ability of a solution to tolerate ph changes when exposed to strong acids or bases, allowing almost all physiological processes in the body to occur. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid.
From www.slideserve.com
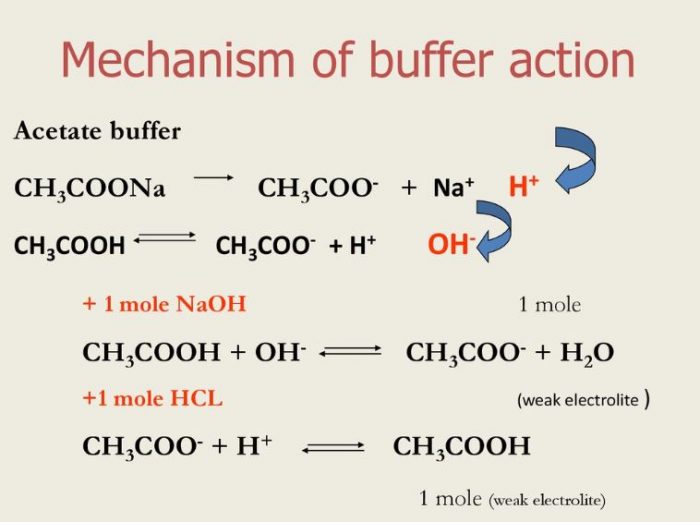
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 What Is A Buffer Medical Term The term buffer was introduced to describe the ability of a solution to tolerate ph changes when exposed to strong acids or bases, allowing almost all physiological processes in the body to occur. Buffers are substances that help maintain the ph of a solution within a specific range. A buffer is a solution that can resist ph change upon the. What Is A Buffer Medical Term.
From www.slideserve.com
PPT Determination of Buffer Capacity PowerPoint Presentation, free What Is A Buffer Medical Term A solution used to stabilize the ph. Buffer solutions consist of a weak acid and its. Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. The four examples of physiological buffers are here. Buffers are substances that help maintain the ph of a solution within a specific range. A buffer is. What Is A Buffer Medical Term.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its What Is A Buffer Medical Term Buffer solutions consist of a weak acid and its. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The four examples of physiological buffers are here. Buffers are substances that help maintain the ph of a solution within a specific range. Physiological buffers are chemicals used by the body to. What Is A Buffer Medical Term.
From www.youtube.com
Buffer Solution Types & Buffer Capacity Pharma What Is A Buffer Medical Term Buffer solutions consist of a weak acid and its. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. A solution used to stabilize the ph. Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. Buffers are solutions that contain a weak acid and its a conjugate. What Is A Buffer Medical Term.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Is A Buffer Medical Term A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. The term buffer was introduced to describe the ability of a solution to tolerate ph changes when exposed to strong acids or bases, allowing almost all physiological processes in the body to occur. A. What Is A Buffer Medical Term.
From www.slideserve.com
PPT What is a buffer? PowerPoint Presentation, free download ID5083921 What Is A Buffer Medical Term A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Buffers are solutions that contain a weak acid and its a conjugate base; A buffer is a solution containing substances which have the ability to minimise changes. What Is A Buffer Medical Term.
From www.slideserve.com
PPT Chemistry 232 PowerPoint Presentation, free download ID845815 What Is A Buffer Medical Term Buffers are substances that help maintain the ph of a solution within a specific range. Buffers are solutions that contain a weak acid and its a conjugate base; The four examples of physiological buffers are here. A solution used to stabilize the ph. A buffer is a solution that can resist ph change upon the addition of an acidic or. What Is A Buffer Medical Term.
From www.youtube.com
HEPES Buffer Good's Buffer Preparation Of HEPES Buffer YouTube What Is A Buffer Medical Term Buffer solutions consist of a weak acid and its. A buffer typically consists of a. A solution used to stabilize the ph. The four examples of physiological buffers are here. Buffers are substances that help maintain the ph of a solution within a specific range. A buffer is a solution containing substances which have the ability to minimise changes in. What Is A Buffer Medical Term.
From www.slideshare.net
Buffer system What Is A Buffer Medical Term Buffers are substances that help maintain the ph of a solution within a specific range. A solution used to stabilize the ph. Buffers are solutions that contain a weak acid and its a conjugate base; A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it. What Is A Buffer Medical Term.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Medical Term Buffer solutions consist of a weak acid and its. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. The four examples of physiological buffers are here. Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. A buffer is a solution that can resist ph change upon. What Is A Buffer Medical Term.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download What Is A Buffer Medical Term The term buffer was introduced to describe the ability of a solution to tolerate ph changes when exposed to strong acids or bases, allowing almost all physiological processes in the body to occur. Buffers are solutions that contain a weak acid and its a conjugate base; A buffer typically consists of a. They work by absorbing or releasing hydrogen ions. What Is A Buffer Medical Term.
From www.chemistrylearner.com
Buffer Solution Definition, Examples, and Preparation What Is A Buffer Medical Term Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. A buffer typically consists of a. Buffers are substances that help maintain the ph of a solution within a specific range. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base. What Is A Buffer Medical Term.
From www.youtube.com
Buffer range and capacity YouTube What Is A Buffer Medical Term Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. The term buffer was introduced to describe the ability of a solution to tolerate ph changes when exposed to strong acids or bases, allowing almost all physiological processes in the body to occur. They work by absorbing or releasing hydrogen ions (h+). What Is A Buffer Medical Term.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID7055849 What Is A Buffer Medical Term Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. A solution used to stabilize the ph. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. A buffer typically consists of a. The four examples of physiological buffers are here. A buffer is a solution containing substances. What Is A Buffer Medical Term.
From www.pinterest.com
Buffer Buffer solution, Easy science, Flashcards What Is A Buffer Medical Term Buffers are solutions that contain a weak acid and its a conjugate base; Buffer solutions consist of a weak acid and its. Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. The term buffer was introduced to describe the ability of a solution to tolerate ph changes when exposed to strong. What Is A Buffer Medical Term.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Is A Buffer Medical Term Buffer solutions consist of a weak acid and its. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. The four examples of physiological buffers are here. A buffer typically consists of a. A buffer is a solution that can resist ph change upon. What Is A Buffer Medical Term.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Is A Buffer Medical Term The term buffer was introduced to describe the ability of a solution to tolerate ph changes when exposed to strong acids or bases, allowing almost all physiological processes in the body to occur. Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. A buffer typically consists of a. They work by. What Is A Buffer Medical Term.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Is A Buffer Medical Term They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. The four examples of physiological buffers are here. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. A buffer is a solution that can resist ph change upon the. What Is A Buffer Medical Term.
From www.youtube.com
BUFFER SOLUTIONS BUFFER CAPACITY APPLICATIONS OF BUFFERS IN What Is A Buffer Medical Term The four examples of physiological buffers are here. A buffer typically consists of a. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Buffer solutions consist of a weak acid and its. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution used to. What Is A Buffer Medical Term.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Medical Term Buffer solutions consist of a weak acid and its. Buffers are solutions that contain a weak acid and its a conjugate base; Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. A buffer typically consists of a. A solution used to stabilize the ph. A buffer is a solution containing substances. What Is A Buffer Medical Term.
From www.brainkart.com
Buffers What Is A Buffer Medical Term Buffers are solutions that contain a weak acid and its a conjugate base; The four examples of physiological buffers are here. Buffer solutions consist of a weak acid and its. The term buffer was introduced to describe the ability of a solution to tolerate ph changes when exposed to strong acids or bases, allowing almost all physiological processes in the. What Is A Buffer Medical Term.
From thenoveldifference.com
Buffer Action and Buffer Capacity The best 4 difference What Is A Buffer Medical Term A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Buffers are solutions that contain a weak acid and its a conjugate base; A solution used to stabilize the ph. The four examples of physiological buffers are. What Is A Buffer Medical Term.
From www.youtube.com
Buffer Capacity 03 Buffers in pharmaceutical and biological systems What Is A Buffer Medical Term A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers are solutions that contain a weak acid and its a conjugate base; A buffer typically consists of a. The four examples of physiological buffers are here. Buffer solutions consist of a weak acid and its. A solution used to stabilize. What Is A Buffer Medical Term.
From www.youtube.com
Buffer System YouTube What Is A Buffer Medical Term A buffer typically consists of a. The four examples of physiological buffers are here. Buffers are solutions that contain a weak acid and its a conjugate base; They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Buffer solutions consist of a weak acid and its. A buffer is a solution that can resist ph change. What Is A Buffer Medical Term.
From loeukppln.blob.core.windows.net
Buffers Ap Biology at Venessa Baird blog What Is A Buffer Medical Term A buffer typically consists of a. The four examples of physiological buffers are here. A solution used to stabilize the ph. Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. A buffer is a solution containing substances. What Is A Buffer Medical Term.
From dxobuticl.blob.core.windows.net
Commonly Used Buffers In The Laboratory at Savannah Osgood blog What Is A Buffer Medical Term The term buffer was introduced to describe the ability of a solution to tolerate ph changes when exposed to strong acids or bases, allowing almost all physiological processes in the body to occur. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. A. What Is A Buffer Medical Term.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high What Is A Buffer Medical Term A buffer typically consists of a. Buffer solutions consist of a weak acid and its. Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The four examples of physiological buffers are here.. What Is A Buffer Medical Term.
From www.youtube.com
BUFFER SOLUTIONS HOW TO MAKE BUFFER ? TYPES OF BUFFER HOW BUFFERS What Is A Buffer Medical Term A buffer typically consists of a. Buffer solutions consist of a weak acid and its. A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.. What Is A Buffer Medical Term.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Is A Buffer Medical Term A buffer is a solution containing substances which have the ability to minimise changes in ph when an acid or base is added to it 1. The term buffer was introduced to describe the ability of a solution to tolerate ph changes when exposed to strong acids or bases, allowing almost all physiological processes in the body to occur. The. What Is A Buffer Medical Term.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Medical Term They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. A buffer typically consists of a. Buffers are solutions that contain a weak acid and its a conjugate base; The term buffer was introduced to describe the ability. What Is A Buffer Medical Term.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Is A Buffer Medical Term A solution used to stabilize the ph. The term buffer was introduced to describe the ability of a solution to tolerate ph changes when exposed to strong acids or bases, allowing almost all physiological processes in the body to occur. A buffer typically consists of a. Buffers are substances that help maintain the ph of a solution within a specific. What Is A Buffer Medical Term.
From www.youtube.com
Acid Base Physiology Part One Basics Buffers Renal Physiology What Is A Buffer Medical Term A buffer typically consists of a. The four examples of physiological buffers are here. Buffers are substances that help maintain the ph of a solution within a specific range. Buffers are solutions that contain a weak acid and its a conjugate base; Buffer solutions consist of a weak acid and its. The term buffer was introduced to describe the ability. What Is A Buffer Medical Term.
From www.youtube.com
Buffer action in the blood YouTube What Is A Buffer Medical Term They work by absorbing or releasing hydrogen ions (h+) to counteract changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A solution used to stabilize the ph. Buffers are substances that help maintain the ph of a solution within a specific range. Physiological buffers are chemicals used. What Is A Buffer Medical Term.
From mydigitalkemistry.com
What is a buffer solution simple definition? Free Chemistry Learning What Is A Buffer Medical Term A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. Buffers are solutions that contain a weak acid and its a conjugate base; The term buffer was introduced to describe the ability of. What Is A Buffer Medical Term.
From exyeghjzx.blob.core.windows.net
What Are The Types Of Buffers at Virginia Saunders blog What Is A Buffer Medical Term A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Physiological buffers are chemicals used by the body to prevent large changes in the ph of bodily fluid. Buffer solutions consist of a weak acid and its. Buffers are solutions that contain a weak acid and its a conjugate base; The. What Is A Buffer Medical Term.