Medical Device Risk Management Report Pdf . In addition to iso 14971, there are several other key medical device industry standards requiring risk. The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. 1 scope management document provides guidance on the development, implementation and maintenance of a risk medical devices on iso. This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in. View of how regulations and standards for medical devices have developed over the recent decades. [1] is discussed in detail and the main. • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities for. Application of risk management to medical devices. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. The risk management process as described in bs en iso 14971.
from sunstonepilot.com
The risk management process as described in bs en iso 14971. • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities for. This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in. Application of risk management to medical devices. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. [1] is discussed in detail and the main. The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. 1 scope management document provides guidance on the development, implementation and maintenance of a risk medical devices on iso. In addition to iso 14971, there are several other key medical device industry standards requiring risk. View of how regulations and standards for medical devices have developed over the recent decades.
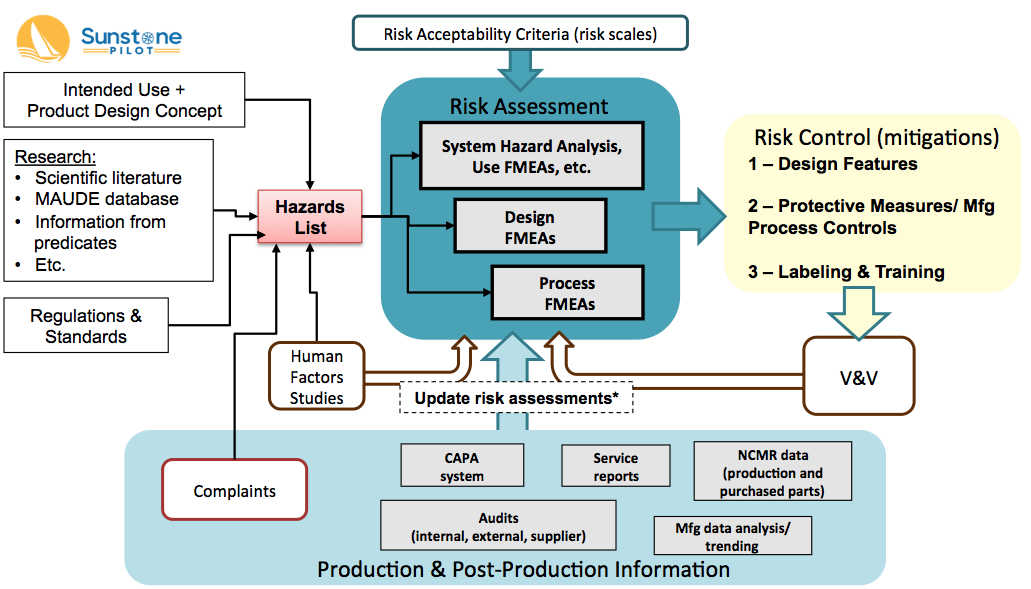
The Big Picture for Medical Device Risk Management Sunstone Pilot, Inc.
Medical Device Risk Management Report Pdf The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities for. The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. 1 scope management document provides guidance on the development, implementation and maintenance of a risk medical devices on iso. The risk management process as described in bs en iso 14971. Application of risk management to medical devices. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. [1] is discussed in detail and the main. This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in. In addition to iso 14971, there are several other key medical device industry standards requiring risk. View of how regulations and standards for medical devices have developed over the recent decades.
From array.aami.org
Documenting Medical Device Risk Management through the Risk Medical Device Risk Management Report Pdf Application of risk management to medical devices. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities for. In addition to iso 14971, there are several other key medical device industry standards requiring risk. The. Medical Device Risk Management Report Pdf.
From sunstonepilot.com
The Big Picture for Medical Device Risk Management Sunstone Pilot, Inc. Medical Device Risk Management Report Pdf In addition to iso 14971, there are several other key medical device industry standards requiring risk. • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities for. The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development. Medical Device Risk Management Report Pdf.
From www.contrapositionmagazine.com
Medical Device Risk Management Report Example Template 2 Resume Medical Device Risk Management Report Pdf The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. Application of risk management to medical devices. 1 scope management document provides guidance on the development, implementation and maintenance of a risk medical devices on iso. • discuss the reasons for conducting risk management activities. Medical Device Risk Management Report Pdf.
From medicaldevicehq.com
FMEA vs ISO 14971 Medical Device HQ 1 Medical Device Risk Management Report Pdf The risk management process as described in bs en iso 14971. [1] is discussed in detail and the main. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in. Application of risk management. Medical Device Risk Management Report Pdf.
From exyppetzm.blob.core.windows.net
Medical Devices Management Policy at Roger Stiles blog Medical Device Risk Management Report Pdf • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities for. The risk management process as described in bs en iso 14971. The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. This document specifies. Medical Device Risk Management Report Pdf.
From www.semanticscholar.org
Case study — Risk management for medical devices (based on ISO 14971 Medical Device Risk Management Report Pdf • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities for. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. [1] is discussed in detail and the main. 1 scope management document provides guidance on the development, implementation and maintenance of a risk medical. Medical Device Risk Management Report Pdf.
From www.orielstat.com
Preparing a Medical Device Risk Management Review and Report Medical Device Risk Management Report Pdf The risk management process as described in bs en iso 14971. [1] is discussed in detail and the main. • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities for. The present focus of risk management in the industry of developing medical devices is on technical risks, such as product,. Medical Device Risk Management Report Pdf.
From www.aplyon.com
Risk Management Procedure Medical Device Risk Management Report Pdf View of how regulations and standards for medical devices have developed over the recent decades. [1] is discussed in detail and the main. This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in. In addition to iso 14971, there are several other key medical device industry standards requiring. Medical Device Risk Management Report Pdf.
From www.smartsheet.com
Free Cybersecurity Risk Assessment Templates Smartsheet Medical Device Risk Management Report Pdf Application of risk management to medical devices. View of how regulations and standards for medical devices have developed over the recent decades. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. The risk management process as described in bs en iso 14971. This document specifies terminology, principles and a process for risk management of. Medical Device Risk Management Report Pdf.
From www.reedtech.com
Medical Device Risk Management FMEA Medical Devices Reed Tech Medical Device Risk Management Report Pdf The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities for. The risk management process as described in bs en iso 14971. This document specifies. Medical Device Risk Management Report Pdf.
From classlesdemocracy.blogspot.com
Risk Management Report Template Classles Democracy Medical Device Risk Management Report Pdf The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. Application of risk management to medical devices. [1] is discussed in detail and the main. View of how regulations and standards for medical devices have developed over the recent decades. The risk management process as. Medical Device Risk Management Report Pdf.
From www.greenlight.guru
Understanding ISO 14971 Medical Device Risk Management Medical Device Risk Management Report Pdf [1] is discussed in detail and the main. This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in. The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. View of how regulations and. Medical Device Risk Management Report Pdf.
From www.pinterest.com
Risk assessment Report Template Luxury Medical Device Risk Management Medical Device Risk Management Report Pdf [1] is discussed in detail and the main. The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in. Application of risk management to. Medical Device Risk Management Report Pdf.
From www.complianceonline.com
ISO 149712019 Medical devices Application of Risk Management to Medical Device Risk Management Report Pdf The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities for. In addition to iso 14971, there are several other key medical device industry standards requiring risk. Application of risk management to medical devices. The. Medical Device Risk Management Report Pdf.
From siamptu.weebly.com
Medical device risk assessment template siamptu Medical Device Risk Management Report Pdf In addition to iso 14971, there are several other key medical device industry standards requiring risk. Application of risk management to medical devices. View of how regulations and standards for medical devices have developed over the recent decades. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. This document specifies terminology, principles and a. Medical Device Risk Management Report Pdf.
From www.orielstat.com
Choosing the right medical device risk management tools Medical Device Risk Management Report Pdf This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in. The risk management process as described in bs en iso 14971. The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. [1] is. Medical Device Risk Management Report Pdf.
From www.presentationeze.com
Medical Devices Risk Management PlanningPresentationEZE Medical Device Risk Management Report Pdf Application of risk management to medical devices. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. View of how regulations and standards for medical devices have developed over the recent decades. [1] is discussed in detail and the main. The risk management process as described in bs en iso 14971. In addition to iso. Medical Device Risk Management Report Pdf.
From dollarsgeser.weebly.com
Medical device risk assessment template dollarsgeser Medical Device Risk Management Report Pdf 1 scope management document provides guidance on the development, implementation and maintenance of a risk medical devices on iso. This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in. [1] is discussed in detail and the main. The present focus of risk management in the industry of developing. Medical Device Risk Management Report Pdf.
From www.childforallseasons.com
Medical Device Risk Management Report Template Template 2 Resume Medical Device Risk Management Report Pdf [1] is discussed in detail and the main. The risk management process as described in bs en iso 14971. • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities for. The present focus of risk management in the industry of developing medical devices is on technical risks, such as product,. Medical Device Risk Management Report Pdf.
From medicaldevicehq.com
Quality Manual Template Part 1 (ISO 13485, Medical Device) Medical Medical Device Risk Management Report Pdf • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities for. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. In addition to iso 14971, there are several other key medical device industry standards requiring risk. Application of risk management to medical devices. 1. Medical Device Risk Management Report Pdf.
From www.pinterest.com
Risk Analysis Module Risk analysis, Analysis, Risk management Medical Device Risk Management Report Pdf The risk management process as described in bs en iso 14971. Application of risk management to medical devices. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. [1] is discussed in detail and the main. 1 scope management document provides guidance on the development, implementation and maintenance of a risk medical devices on iso.. Medical Device Risk Management Report Pdf.
From sunstonepilot.com
The Big Picture for Medical Device Risk Management Sunstone Pilot, Inc. Medical Device Risk Management Report Pdf 1 scope management document provides guidance on the development, implementation and maintenance of a risk medical devices on iso. The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. [1] is discussed in detail and the main. • discuss the reasons for conducting risk management. Medical Device Risk Management Report Pdf.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials Medical Device Risk Management Report Pdf Application of risk management to medical devices. In addition to iso 14971, there are several other key medical device industry standards requiring risk. The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. 1 scope management document provides guidance on the development, implementation and maintenance. Medical Device Risk Management Report Pdf.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials Medical Device Risk Management Report Pdf This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. Application of risk management to medical devices. In addition to iso 14971, there are several other key medical device industry standards requiring risk.. Medical Device Risk Management Report Pdf.
From starfishmedical.com
Medical Device Risk Management Medical Device Risk Management Report Pdf [1] is discussed in detail and the main. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. Application of risk management to medical devices. In addition to iso 14971, there are several other key medical device industry standards requiring risk. 1 scope management document provides guidance on the development, implementation and maintenance of a. Medical Device Risk Management Report Pdf.
From www.meddeviceonline.com
Managing Risk For Medical Device Clinical Trials Medical Device Risk Management Report Pdf The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. Application of risk management to medical devices. [1] is discussed in detail and the main. • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities. Medical Device Risk Management Report Pdf.
From www.childforallseasons.com
Medical Device Risk Management Report Template Template 2 Resume Medical Device Risk Management Report Pdf • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities for. 1 scope management document provides guidance on the development, implementation and maintenance of a risk medical devices on iso. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. [1] is discussed in detail. Medical Device Risk Management Report Pdf.
From www.scribd.com
88 NailRisk Management Report PDF PDF Medical Device Risk Medical Device Risk Management Report Pdf This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in. In addition to iso 14971, there are several other key medical device industry standards requiring risk. The risk management process as described in bs en iso 14971. 1 scope management document provides guidance on the development, implementation and. Medical Device Risk Management Report Pdf.
From www.cybersaint.io
Risk Register Examples for Cybersecurity Leaders Medical Device Risk Management Report Pdf • discuss the reasons for conducting risk management activities for medical devices • identify when to use risk management activities for. [1] is discussed in detail and the main. This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in. The requirements of iso 14971:20072019, medical devices — application. Medical Device Risk Management Report Pdf.
From www.contrapositionmagazine.com
Medical Device Risk Management Report Template Template 2 Resume Medical Device Risk Management Report Pdf This document specifies terminology, principles and a process for risk management of medical devices, including software as a medical device and in. The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. • discuss the reasons for conducting risk management activities for medical devices •. Medical Device Risk Management Report Pdf.
From www.youtube.com
Risk Management in the medical device industry in the EU YouTube Medical Device Risk Management Report Pdf The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. In addition to iso 14971, there are several other key medical device industry standards requiring risk. 1 scope management document provides guidance on the development, implementation and maintenance of a risk medical devices on iso. View of how regulations and standards for medical devices have. Medical Device Risk Management Report Pdf.
From medicaldevicehq.com
How to integrate proactive safety by design with medical device risk Medical Device Risk Management Report Pdf The risk management process as described in bs en iso 14971. View of how regulations and standards for medical devices have developed over the recent decades. 1 scope management document provides guidance on the development, implementation and maintenance of a risk medical devices on iso. • discuss the reasons for conducting risk management activities for medical devices • identify when. Medical Device Risk Management Report Pdf.
From www.orielstat.com
Creating a Medical Device Risk Management Plan and Doing Analysis Medical Device Risk Management Report Pdf View of how regulations and standards for medical devices have developed over the recent decades. The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. Application of risk management to medical devices. This document specifies terminology, principles and a process for risk management of medical. Medical Device Risk Management Report Pdf.
From www.examples.com
Risk Register 46+ Examples, Format, Pdf Medical Device Risk Management Report Pdf The present focus of risk management in the industry of developing medical devices is on technical risks, such as product, usability, and development process hazards. 1 scope management document provides guidance on the development, implementation and maintenance of a risk medical devices on iso. View of how regulations and standards for medical devices have developed over the recent decades. The. Medical Device Risk Management Report Pdf.
From denner-shop-test-web02.denner.ch
Quality Plan Template Medical Device Medical Device Risk Management Report Pdf [1] is discussed in detail and the main. The risk management process as described in bs en iso 14971. The requirements of iso 14971:20072019, medical devices — application of risk management to medical devices. 1 scope management document provides guidance on the development, implementation and maintenance of a risk medical devices on iso. Application of risk management to medical devices.. Medical Device Risk Management Report Pdf.