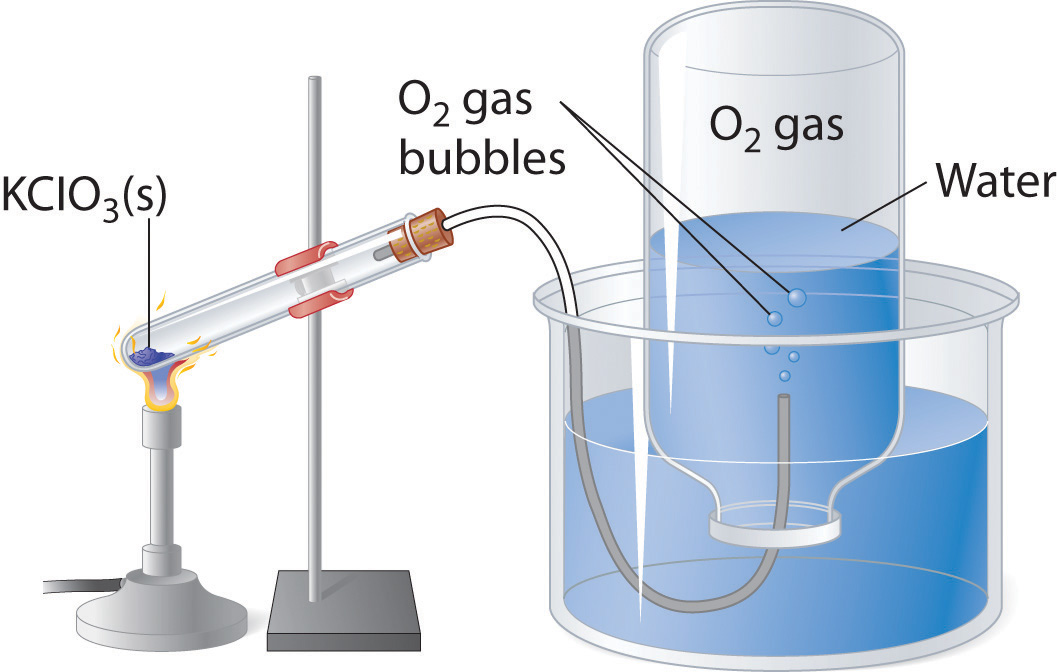
Gas Law Experiment Examples . In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry. It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. Boyle’s law explains the relationship between the pressure and volume of a perfect gas. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Inside our container, the temperature,. A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining one the same. The overall aim of this experiment is to investigate charles’s law, which is the effect of temperature on volume at constant. Imagine we have a container filled with gas, for example, air, as it will be the case in this experiment.
from chem.libretexts.org
Boyle’s law explains the relationship between the pressure and volume of a perfect gas. It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. The overall aim of this experiment is to investigate charles’s law, which is the effect of temperature on volume at constant. In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry. Imagine we have a container filled with gas, for example, air, as it will be the case in this experiment. A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining one the same. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Inside our container, the temperature,.
8.5 Gas Laws Chemistry LibreTexts
Gas Law Experiment Examples Boyle’s law explains the relationship between the pressure and volume of a perfect gas. A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining one the same. Inside our container, the temperature,. Boyle’s law explains the relationship between the pressure and volume of a perfect gas. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Imagine we have a container filled with gas, for example, air, as it will be the case in this experiment. In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry. It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. The overall aim of this experiment is to investigate charles’s law, which is the effect of temperature on volume at constant.
From chem.libretexts.org
Chapter 6.4 The Combined Gas Law Chemistry LibreTexts Gas Law Experiment Examples A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining one the same. Boyle’s law explains the relationship between the pressure and volume of a perfect gas. Inside our container, the temperature,. A gas will act like an ideal gas if its gas molecules are. Gas Law Experiment Examples.
From www.youtube.com
5 Ideal Gas Law Experiments PV=nRT or PV=NkT YouTube Gas Law Experiment Examples In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry. The overall aim of this experiment is to investigate charles’s law, which is the effect of temperature on volume at constant. A gas will act like an ideal gas if its gas molecules are small, when the pressure. Gas Law Experiment Examples.
From studiousguy.com
8 Boyle’s Law Examples in Real Life StudiousGuy Gas Law Experiment Examples It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. The overall aim of this experiment is to investigate charles’s law, which is the effect of temperature on volume at constant. Imagine we have a container filled with gas, for example, air, as it will be the case in this experiment. A gas. Gas Law Experiment Examples.
From www.pearson.com
5 Ideal Gas Law Experiments PV=nRT or PV=NkT Pearson+ Channels Gas Law Experiment Examples Boyle’s law explains the relationship between the pressure and volume of a perfect gas. It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. Imagine we have a container filled with gas, for example, air, as it will be the case in this experiment. In this experiment, you will generate a sample of. Gas Law Experiment Examples.
From flashyscience.com
Gas Laws Experiments FlashyScience Gas Law Experiment Examples Boyle’s law explains the relationship between the pressure and volume of a perfect gas. In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry. It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. A gas law experiment shows what happens. Gas Law Experiment Examples.
From chemistryteory.blogspot.com
chemistry Gas Laws Boyle’s Law Gas Law Experiment Examples Boyle’s law explains the relationship between the pressure and volume of a perfect gas. The overall aim of this experiment is to investigate charles’s law, which is the effect of temperature on volume at constant. A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining. Gas Law Experiment Examples.
From sciencenotes.org
Ideal Gas Law Formula and Examples Gas Law Experiment Examples A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. Boyle’s law explains the relationship between the pressure and volume of a perfect gas. Inside our container, the temperature,.. Gas Law Experiment Examples.
From www.pinterest.com
Charles’s Gas Law a fun experiment involving a glass soda bottle and a balloon http Gas Law Experiment Examples In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry. It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature,. Gas Law Experiment Examples.
From sciencenotes.org
Boyle's Law Definition, Formula, Example Gas Law Experiment Examples A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining one the same. Boyle’s law explains the relationship between the pressure and volume of a perfect gas. It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. Imagine. Gas Law Experiment Examples.
From www.showme.com
Ideal gas law example Science, Chemistry, Gases ShowMe Gas Law Experiment Examples A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining one the same. In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry. Imagine we have a container filled with gas, for example,. Gas Law Experiment Examples.
From www.academia.edu
(PDF) EXPERIMENT 5 IDEAL GAS LAW CHARLES'S LAW Priya Sundaraju Academia.edu Gas Law Experiment Examples Boyle’s law explains the relationship between the pressure and volume of a perfect gas. Imagine we have a container filled with gas, for example, air, as it will be the case in this experiment. Inside our container, the temperature,. In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using. Gas Law Experiment Examples.
From www.youtube.com
gas law lab experiment 1 YouTube Gas Law Experiment Examples The overall aim of this experiment is to investigate charles’s law, which is the effect of temperature on volume at constant. A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining one the same. Inside our container, the temperature,. Boyle’s law explains the relationship between. Gas Law Experiment Examples.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Gas Law Experiment Examples A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining one the same. It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. In this experiment, you will generate a sample of gas chemically, so that you can. Gas Law Experiment Examples.
From www.tec-science.com
Ideal gas law (explained and derived) tecscience Gas Law Experiment Examples It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. In this experiment, you will generate a sample of gas chemically, so that you can determine the number of. Gas Law Experiment Examples.
From www.britannica.com
perfect gas law chemistry and physics Britannica Gas Law Experiment Examples A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining one the same. Imagine we have a container filled with gas, for example, air, as it will be the case in this experiment. It states that the volume of a perfect gas is inversely proportional. Gas Law Experiment Examples.
From www.chegg.com
Using the ideal Gas Law EXPERIMENT 2 USING THE IDEAL Gas Law Experiment Examples It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. Imagine we have a container filled with gas, for example, air, as it will be the case in this experiment. The overall aim of this experiment is to investigate charles’s law, which is the effect of temperature on volume at constant. Boyle’s law. Gas Law Experiment Examples.
From www.youtube.com
Chapter 7 Lecture 4 Combined Gas Law YouTube Gas Law Experiment Examples It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the. Gas Law Experiment Examples.
From studylib.net
Common Gas Law Experiments Collapsing Balloon (Charles' Law) Gas Law Experiment Examples Imagine we have a container filled with gas, for example, air, as it will be the case in this experiment. A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining one the same. The overall aim of this experiment is to investigate charles’s law, which. Gas Law Experiment Examples.
From www.chegg.com
The Ideal Gas Law EXPERIMENT 1 CHARLES'S LAW Data Gas Law Experiment Examples The overall aim of this experiment is to investigate charles’s law, which is the effect of temperature on volume at constant. Imagine we have a container filled with gas, for example, air, as it will be the case in this experiment. A gas law experiment shows what happens to one property, such as the volume, when you change another, such. Gas Law Experiment Examples.
From www.youtube.com
Ideal Gas Law Experiment YouTube Gas Law Experiment Examples A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Boyle’s law explains the relationship between the pressure and volume of a perfect gas. In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry.. Gas Law Experiment Examples.
From www.scribd.com
Chem Lab Report 9 (2)Gas Law Gases Temperature Gas Law Experiment Examples A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Inside our container, the temperature,. In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry. The overall aim of this experiment is to investigate. Gas Law Experiment Examples.
From artofteachingscience.blogspot.com
The Art of Teaching Science Gas Laws Where it all Began Gas Law Experiment Examples A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Imagine we have a container filled with gas, for example, air, as it will be the case in this experiment. A gas law experiment shows what happens to one property, such as the volume, when you. Gas Law Experiment Examples.
From www.youtube.com
IDEAL GAS LAW PRACTICE PROBLEMS How to Solve Ideal Gas Law Problems in Chemistry YouTube Gas Law Experiment Examples In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry. Inside our container, the temperature,. A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining one the same. The overall aim of this. Gas Law Experiment Examples.
From www.britannica.com
Boyle’s law Definition, Equation, & Facts Britannica Gas Law Experiment Examples A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. A gas law experiment shows what happens to one property, such as the volume, when you change another, such. Gas Law Experiment Examples.
From studiousguy.com
The Gas Laws Definition, Formula & Examples StudiousGuy Gas Law Experiment Examples The overall aim of this experiment is to investigate charles’s law, which is the effect of temperature on volume at constant. Inside our container, the temperature,. A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining one the same. It states that the volume of. Gas Law Experiment Examples.
From www.dreamstime.com
Charles Law Infographic Diagram Example Expand Heat Compress Cold Ideal Gas Experiment Stock Gas Law Experiment Examples A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. Inside our container, the temperature,. A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature, while keeping the remaining one the same. Imagine we have. Gas Law Experiment Examples.
From www.youtube.com
Gases Gas Stoichiometry & Dalton's Law. YouTube Gas Law Experiment Examples A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry. A gas law experiment shows what happens to one property, such as the volume,. Gas Law Experiment Examples.
From www.youtube.com
Avogadro's law Avogadro's gas law Experiment YouTube Gas Law Experiment Examples Imagine we have a container filled with gas, for example, air, as it will be the case in this experiment. The overall aim of this experiment is to investigate charles’s law, which is the effect of temperature on volume at constant. It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. Inside our. Gas Law Experiment Examples.
From www.pearson.com
5 Ideal Gas Law Experiments PV=nRT or PV=NkT Pearson+ Channels Gas Law Experiment Examples In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry. The overall aim of this experiment is to investigate charles’s law, which is the effect of temperature on volume at constant. Imagine we have a container filled with gas, for example, air, as it will be the case. Gas Law Experiment Examples.
From www.youtube.com
Ideal Gas Law Home Experiment YouTube Gas Law Experiment Examples Inside our container, the temperature,. Boyle’s law explains the relationship between the pressure and volume of a perfect gas. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. A gas law experiment shows what happens to one property, such as the volume, when you change. Gas Law Experiment Examples.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock Vector Illustration of Gas Law Experiment Examples In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry. Imagine we have a container filled with gas, for example, air, as it will be the case in this experiment. Boyle’s law explains the relationship between the pressure and volume of a perfect gas. The overall aim of. Gas Law Experiment Examples.
From www.youtube.com
IDEAL GAS LAW CHEMISTRY TUTORIAL SCIENCE LESSON YouTube Gas Law Experiment Examples Inside our container, the temperature,. Boyle’s law explains the relationship between the pressure and volume of a perfect gas. Imagine we have a container filled with gas, for example, air, as it will be the case in this experiment. A gas law experiment shows what happens to one property, such as the volume, when you change another, such as temperature,. Gas Law Experiment Examples.
From chem.libretexts.org
8.5 Gas Laws Chemistry LibreTexts Gas Law Experiment Examples The overall aim of this experiment is to investigate charles’s law, which is the effect of temperature on volume at constant. Inside our container, the temperature,. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high. In this experiment, you will generate a sample of gas. Gas Law Experiment Examples.
From www.youtube.com
Charles' Law, a subset of the ideal gas law. burning candle activity YouTube Gas Law Experiment Examples It states that the volume of a perfect gas is inversely proportional to the absolute pressure, provided. Inside our container, the temperature,. Boyle’s law explains the relationship between the pressure and volume of a perfect gas. The overall aim of this experiment is to investigate charles’s law, which is the effect of temperature on volume at constant. A gas will. Gas Law Experiment Examples.
From www.youtube.com
Experiment 9 Gas Laws SMU Chemistry YouTube Gas Law Experiment Examples In this experiment, you will generate a sample of gas chemically, so that you can determine the number of moles using stoichiometry. Boyle’s law explains the relationship between the pressure and volume of a perfect gas. A gas will act like an ideal gas if its gas molecules are small, when the pressure is low, and the temperature is high.. Gas Law Experiment Examples.