Medical Device Cleaning Validation . The training will include examples from cleaning validations and test method investigations. It replaces aami tir30 and provides requirements to. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Ansi/aami st98:2022 is a new, published standard. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean.
from www.orielstat.com
Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. It replaces aami tir30 and provides requirements to. Ansi/aami st98:2022 is a new, published standard. The training will include examples from cleaning validations and test method investigations.
Medical Device Process Validation Overview & Steps Oriel STAT A MATRIX
Medical Device Cleaning Validation The training will include examples from cleaning validations and test method investigations. The training will include examples from cleaning validations and test method investigations. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Ansi/aami st98:2022 is a new, published standard. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. It replaces aami tir30 and provides requirements to.
From optisolve.net
Medical Facility Cleaning Validation Optisolve Medical Device Cleaning Validation Ansi/aami st98:2022 is a new, published standard. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. The training will include examples from cleaning validations and test method investigations. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. Provide guidance to medical device manufacturers in. Medical Device Cleaning Validation.
From highpowervtls.com
Challenges in Medical Device Cleaning Validation Medical Device Cleaning Validation Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. The training will include examples from cleaning validations and test method investigations. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. Ansi/aami st98:2022 is a new, published standard. Validation of cleaning procedures has. Medical Device Cleaning Validation.
From eranyona.com
Medical Device Cleaning Techniques and Validation Eran Yona Medical Device Cleaning Validation Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. It replaces aami tir30 and provides requirements to. The training will include examples from cleaning validations and test method investigations. Ansi/aami st98:2022 is a new, published standard. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection. Medical Device Cleaning Validation.
From www.youtube.com
Guidance for Cleaning, Disinfection and Sterilization of Reusable Medical Devices YouTube Medical Device Cleaning Validation Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. It replaces aami tir30 and provides requirements to. Ansi/aami st98:2022 is a new, published standard. The training will include examples from cleaning validations and test method. Medical Device Cleaning Validation.
From www.one-eighty-degrees.com
Medical Device Cleaning Validation One Eighty Degrees Medical Device Cleaning Validation Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. The training will include examples from cleaning validations and test method investigations. It replaces aami tir30 and provides requirements to. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. Ansi/aami st98:2022 is a new, published. Medical Device Cleaning Validation.
From easymedicaldevice.com
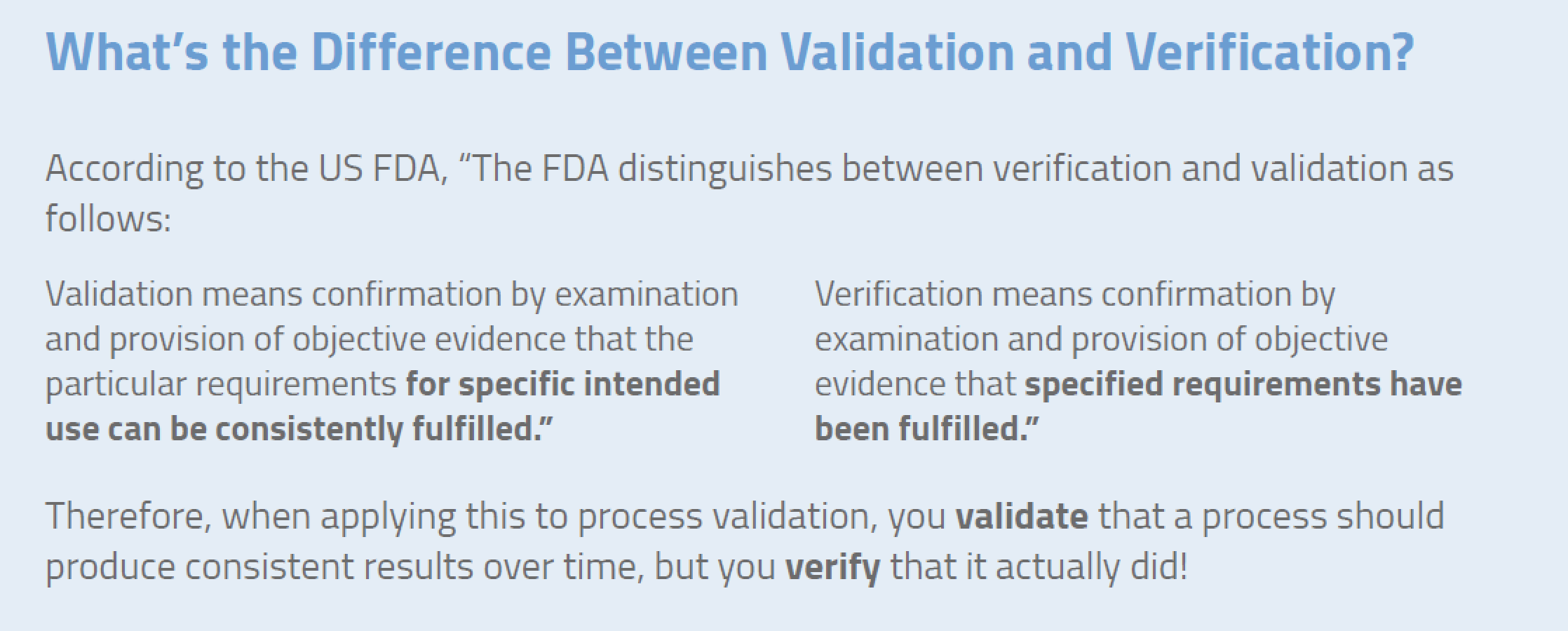
Process Validation or Verification (Medical Device)? Medical Device Cleaning Validation It replaces aami tir30 and provides requirements to. The training will include examples from cleaning validations and test method investigations. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Ansi/aami st98:2022 is a new, published standard. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices. Medical Device Cleaning Validation.
From www.aplyon.com
Medical Device Process Validation Procedure ISO 13485 and FDA QSR Compliant Medical Device Cleaning Validation Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. Ansi/aami st98:2022 is a new, published standard. The training will include examples from cleaning validations and test method investigations. Provide guidance to medical device manufacturers in. Medical Device Cleaning Validation.
From highpowervtls.com
Medical Device Cleaning vs. Sterilization Validation Medical Device Cleaning Validation Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. It replaces aami tir30 and provides requirements to. The training will include examples from cleaning validations and test method investigations. Ansi/aami st98:2022 is a new, published standard. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for. Medical Device Cleaning Validation.
From pacificbiolabs.com
Reusable Device Cleaning Validation Three Cycles or One Cycle? Pacific BioLabs Medical Device Cleaning Validation Ansi/aami st98:2022 is a new, published standard. It replaces aami tir30 and provides requirements to. The training will include examples from cleaning validations and test method investigations. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are. Medical Device Cleaning Validation.
From eranyona.com
Cleaning Validation for BioPharmaceutical and Medical Device Eran Yona Medical Device Cleaning Validation Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. It replaces aami tir30 and provides requirements to. The training will include examples from cleaning validations and test method investigations. Ansi/aami st98:2022 is a new, published standard. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are. Medical Device Cleaning Validation.
From www.lucideon.com
Medical Device Cleaning Method Validation to ANSI/AAMI ST98 Requirements Lucideon Medical Device Cleaning Validation Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. It replaces aami tir30 and provides requirements to. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Ansi/aami st98:2022 is a new, published standard. Cleaning validation is documentation establishing that a cleaning process. Medical Device Cleaning Validation.
From www.medtech-expo.ch
Tests within the scope of validation of cleaning of medical devices and materials Medical Device Cleaning Validation Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. The training will include examples from cleaning validations and test method investigations. Provide guidance to medical device manufacturers in the complex activities involved in crafting and. Medical Device Cleaning Validation.
From www.researchgate.net
(PDF) Cleaning Validation of medical products Medical Device Cleaning Validation Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. It replaces aami tir30 and provides requirements to. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. The training will include examples from cleaning validations and test method investigations. Provide guidance to medical device manufacturers. Medical Device Cleaning Validation.
From www.linkedin.com
Test Labs Medical Devices on LinkedIn Medical Device Cleaning Validation Test Labs Medical Device Cleaning Validation It replaces aami tir30 and provides requirements to. The training will include examples from cleaning validations and test method investigations. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. Ansi/aami st98:2022 is a new, published standard. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing. Medical Device Cleaning Validation.
From www.ttslaboratuvar.com
Cleaning Validation of Reusable Medical Devices Blog True Testing Services Medical Device Cleaning Validation Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. It replaces aami tir30 and provides requirements to. Ansi/aami st98:2022 is a new, published standard. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. Validation of cleaning procedures has generated considerable discussion since. Medical Device Cleaning Validation.
From www.scitech.com
Pharmaceutical, Medical Device Cleaning Validation Scitech Medical Device Cleaning Validation It replaces aami tir30 and provides requirements to. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Ansi/aami st98:2022 is a new, published standard. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. The training will include examples from cleaning validations and. Medical Device Cleaning Validation.
From eranyona.com
Medical Devices Cleaning Validation Eran Yona Medical Device Cleaning Validation The training will include examples from cleaning validations and test method investigations. It replaces aami tir30 and provides requirements to. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. Ansi/aami st98:2022 is a new, published standard. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are. Medical Device Cleaning Validation.
From www.researchgate.net
(PDF) Cleaning and Cleaning Validation during medical device development Medical Device Cleaning Validation Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. The training will include examples from cleaning validations and test method investigations. Ansi/aami st98:2022 is a new, published standard. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. Cleaning validation is documentation establishing. Medical Device Cleaning Validation.
From www.cirs-ck.com
Cleaning, Disinfection, Sterilization Process Validation Medical Devices C&K Testing Medical Device Cleaning Validation Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. The training will include examples from cleaning validations and test method investigations. Ansi/aami st98:2022 is a new, published standard. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. It replaces aami tir30 and. Medical Device Cleaning Validation.
From www.regdesk.co
FDA Guidance on Reprocessing Medical Devices Validation of Cleaning RegDesk Medical Device Cleaning Validation Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. It replaces aami tir30 and provides requirements to. Ansi/aami st98:2022 is a new, published standard. The training will include examples from cleaning validations and test method investigations. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for. Medical Device Cleaning Validation.
From www.scribd.com
Cleanliness Validation White Paper Medical Device PDF Federal Food Raman Spectroscopy Medical Device Cleaning Validation The training will include examples from cleaning validations and test method investigations. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. Cleaning validation is documentation establishing that a cleaning process will consistently result. Medical Device Cleaning Validation.
From pharmastate.academy
Cleaning Validation in Pharmaceutical Industry Medical Device Cleaning Validation Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. The training will include examples from cleaning validations and test method investigations. Ansi/aami st98:2022 is a new, published standard. It replaces aami tir30 and provides requirements to. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing. Medical Device Cleaning Validation.
From htwlab.com
medical device cleaning and disinfection sterilization verification Medical Device Cleaning Validation It replaces aami tir30 and provides requirements to. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Ansi/aami st98:2022 is a new, published standard. Validation of cleaning procedures has generated considerable discussion since. Medical Device Cleaning Validation.
From www.youtube.com
Key Factors that Determine a Successful Reusable Medical Device Cleaning Validation YouTube Medical Device Cleaning Validation Ansi/aami st98:2022 is a new, published standard. It replaces aami tir30 and provides requirements to. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. The training will include examples from cleaning validations and test method investigations. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection. Medical Device Cleaning Validation.
From www.scribd.com
Cleaning Validation For Medical Device Manufacturing PDF Verification And Validation Toxicity Medical Device Cleaning Validation Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Ansi/aami st98:2022 is a new, published standard. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. It replaces aami tir30 and provides requirements to. Validation of cleaning procedures has generated considerable discussion since. Medical Device Cleaning Validation.
From www.pacificbiolabs.com
Medical Device Cleaning Validation and Disinfection Validation Pacific BioLabs Medical Device Cleaning Validation It replaces aami tir30 and provides requirements to. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that. Medical Device Cleaning Validation.
From www.scribd.com
Validation of A Cleaning Process For Medical Devices PDF Verification And Validation Medical Device Cleaning Validation Ansi/aami st98:2022 is a new, published standard. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk.. Medical Device Cleaning Validation.
From array.aami.org
Cleaning validation of health care products—Requirements for development and validation of a Medical Device Cleaning Validation The training will include examples from cleaning validations and test method investigations. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. It replaces aami tir30 and provides requirements to. Validation of cleaning procedures. Medical Device Cleaning Validation.
From vdocuments.site
Cleaning Validation for Medical Device Manufacturing · Cleaning Validation for Medical Device Medical Device Cleaning Validation Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. Ansi/aami st98:2022 is a new, published standard. It replaces aami tir30 and provides requirements to. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. The training will include examples from cleaning validations and test method. Medical Device Cleaning Validation.
From www.getreskilled.com
What is Cleaning Validation? GetReskilled Medical Device Cleaning Validation It replaces aami tir30 and provides requirements to. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. Ansi/aami st98:2022 is a new, published standard. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. The training will include examples from cleaning validations and test method. Medical Device Cleaning Validation.
From technotes.alconox.com
Medical Device Cleaning Validation References TechNotes Critical Cleaning Advice from Medical Device Cleaning Validation Ansi/aami st98:2022 is a new, published standard. The training will include examples from cleaning validations and test method investigations. It replaces aami tir30 and provides requirements to. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing. Medical Device Cleaning Validation.
From www.lucideon.com
Medical and Orthopaedic Device Cleaning and Sterilisation Validation Lucideon Medical Device Cleaning Validation Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. It replaces aami tir30 and provides requirements to. Ansi/aami st98:2022 is a new, published standard. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Validation of cleaning procedures has generated considerable discussion since. Medical Device Cleaning Validation.
From www.orielstat.com
Medical Device Process Validation Overview & Steps Oriel STAT A MATRIX Medical Device Cleaning Validation Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. The training will include examples from cleaning. Medical Device Cleaning Validation.
From www.cfpie.com
Cleaning Validation Guidelines The Basics You Need to Know Medical Device Cleaning Validation The training will include examples from cleaning validations and test method investigations. It replaces aami tir30 and provides requirements to. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that ensure. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. Ansi/aami st98:2022 is a. Medical Device Cleaning Validation.
From pacificbiolabs.com
Medical Device Cleaning Validation and Disinfection Validation Pacific BioLabs Medical Device Cleaning Validation It replaces aami tir30 and provides requirements to. Cleaning validation is documentation establishing that a cleaning process will consistently result in devices that are clean. Validation of cleaning procedures has generated considerable discussion since agency documents, including the inspection guide for bulk. Provide guidance to medical device manufacturers in the complex activities involved in crafting and validating reprocessing instructions that. Medical Device Cleaning Validation.