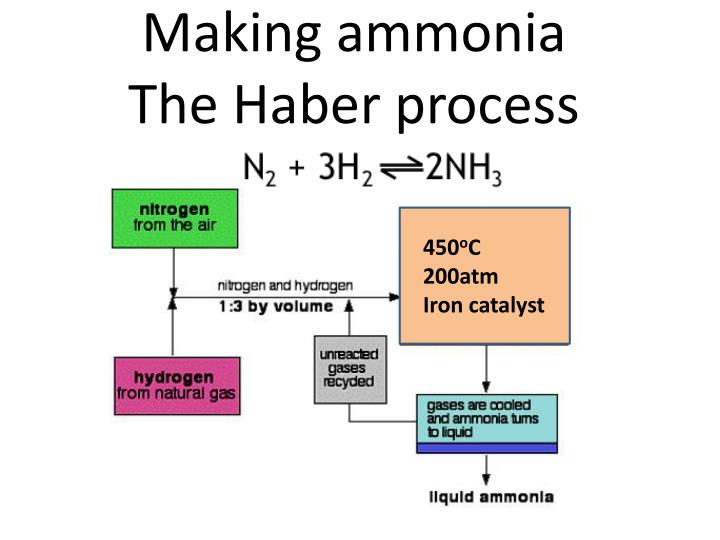
Reaction Vessel Ammonia . N 2 (g) + 3h 2 (g) → 2nh 3 (g) The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process. In these conditions, some of the hydrogen and nitrogen will react to form ammonia. The reaction is reversible and the production of ammonia is. The haber process consists of putting together n2 and h2 in a high. The chemical reaction is given below. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. The haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. A famous equilibrium reaction is the haber process for synthesizing ammonia.
from www.slideserve.com
N 2 (g) + 3h 2 (g) → 2nh 3 (g) The haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. A famous equilibrium reaction is the haber process for synthesizing ammonia. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The haber process consists of putting together n2 and h2 in a high. The chemical reaction is given below. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process. In these conditions, some of the hydrogen and nitrogen will react to form ammonia.
PPT Making ammonia The Haber process PowerPoint Presentation ID2054016
Reaction Vessel Ammonia Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. The chemical reaction is given below. N 2 (g) + 3h 2 (g) → 2nh 3 (g) The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. The haber process consists of putting together n2 and h2 in a high. A famous equilibrium reaction is the haber process for synthesizing ammonia. In these conditions, some of the hydrogen and nitrogen will react to form ammonia. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. The haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. The reaction is reversible and the production of ammonia is. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process.
From www.indiamart.com
Stainless Steel Ammonia Pressure Vessel, Capacity 100500 L at Rs 25000/unit in Pune Reaction Vessel Ammonia The haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. A famous equilibrium reaction is the haber process for synthesizing ammonia. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. The reaction is reversible and the production of ammonia is. N 2 (g) +. Reaction Vessel Ammonia.
From www.indiamart.com
Asha Enterprises Mild Steel Ammonia Pressure Vessels, Max Design Pressure 30 bar, Capacity Reaction Vessel Ammonia The haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. N 2 (g) + 3h 2 (g) → 2nh 3 (g) The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. In these conditions, some of the hydrogen and nitrogen will react to form ammonia.. Reaction Vessel Ammonia.
From ammoniaknowhow.com
Improving the Operation of Ammonia Synthesis Loops AmmoniaKnowHow Reaction Vessel Ammonia The haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. N 2 (g) + 3h 2 (g) → 2nh 3 (g). Reaction Vessel Ammonia.
From www.numerade.com
SOLVEDAmmonia reacts with oxygen to yield nitrogen and water. 4 NH3(g)+3 O2(g) →2 N2(g)+6 H2 O Reaction Vessel Ammonia The reaction is reversible and the production of ammonia is. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. N 2 (g). Reaction Vessel Ammonia.
From www.chegg.com
Solved Nitrogen monoxide and water react to form ammonia and Reaction Vessel Ammonia The haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. A famous equilibrium reaction is the haber process for synthesizing ammonia. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy.. Reaction Vessel Ammonia.
From instrumentationtools.com
Questions on Chemical Reactor Vessel P & ID InstrumentationTools Reaction Vessel Ammonia In these conditions, some of the hydrogen and nitrogen will react to form ammonia. The reaction is reversible and the production of ammonia is. N 2 (g) + 3h 2 (g) → 2nh 3 (g) The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions. Reaction Vessel Ammonia.
From smardt-pressure-vessels.com
Ammonia vessels Smardt Pressure Vessels Reaction Vessel Ammonia N 2 (g) + 3h 2 (g) → 2nh 3 (g) The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. Notably, in this process, the reaction is an exothermic reaction one where there is a release of. Reaction Vessel Ammonia.
From www.numerade.com
SOLVEDIf 2.00 moles of nitrogen and 5.50 moles of hydrogen are placed in a reaction vessel and Reaction Vessel Ammonia A famous equilibrium reaction is the haber process for synthesizing ammonia. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The reaction. Reaction Vessel Ammonia.
From www.chegg.com
Solved Nitrogen and hydrogen react to form ammonia, like Reaction Vessel Ammonia A famous equilibrium reaction is the haber process for synthesizing ammonia. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process. The chemical. Reaction Vessel Ammonia.
From www.azom.com
Ammonia Production Control Reaction Vessel Ammonia N 2 (g) + 3h 2 (g) → 2nh 3 (g) Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The chemical reaction is given below. The haber process is used in the. Reaction Vessel Ammonia.
From dokumen.tips
(PPT) Consider the reaction for the formation of ammonia from nitrogen and hydrogen. I. What is Reaction Vessel Ammonia In these conditions, some of the hydrogen and nitrogen will react to form ammonia. The chemical reaction is given below. The haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for. Reaction Vessel Ammonia.
From edrawsoft.com
Ammonia and Water Reactions Free Ammonia and Water Reactions Templates Reaction Vessel Ammonia N 2 (g) + 3h 2 (g) → 2nh 3 (g) A famous equilibrium reaction is the haber process for synthesizing ammonia. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The chemical reaction is given below. The reaction is reversible and the production of ammonia is. The haber process consists. Reaction Vessel Ammonia.
From www.tradewindsnews.com
MOL presses ahead with ammoniafuelled newcastlemax bulker TradeWinds Reaction Vessel Ammonia The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process. The chemical reaction is given below. The haber process consists of putting together n2 and h2 in a high. The haber process combines nitrogen from the air with hydrogen derived mainly. Reaction Vessel Ammonia.
From www.wikihow.life
3 Ways to Study the Chemical Reactions of Ammonia wikiHow Life Reaction Vessel Ammonia N 2 (g) + 3h 2 (g) → 2nh 3 (g) The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The haber process consists of putting together n2 and h2 in a high. The chemical reaction is. Reaction Vessel Ammonia.
From www.researchgate.net
Ammonia storage and liquefaction process Download Scientific Diagram Reaction Vessel Ammonia The reaction is reversible and the production of ammonia is. A famous equilibrium reaction is the haber process for synthesizing ammonia. The haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. N 2 (g) + 3h 2 (g) → 2nh 3 (g) The haber process combines nitrogen from the air with hydrogen derived. Reaction Vessel Ammonia.
From www.feconsult.com
drafting_Vessel_Ammonia FE Consultants Reaction Vessel Ammonia A famous equilibrium reaction is the haber process for synthesizing ammonia. The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. The chemical reaction is given below. N 2 (g) + 3h 2 (g) → 2nh 3 (g) In these conditions, some of the hydrogen and nitrogen will react to form ammonia. The haber process is. Reaction Vessel Ammonia.
From chempedia.info
Ammonia synthesis converter details Big Chemical Encyclopedia Reaction Vessel Ammonia In these conditions, some of the hydrogen and nitrogen will react to form ammonia. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into. Reaction Vessel Ammonia.
From www.chegg.com
Solved A reaction vessel for synthesizing ammonia by Reaction Vessel Ammonia The haber process consists of putting together n2 and h2 in a high. The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. In these conditions, some of the hydrogen and nitrogen will react to form ammonia. The chemical reaction is given below. N 2 (g) + 3h 2 (g) → 2nh 3 (g) A famous. Reaction Vessel Ammonia.
From www.researchgate.net
Ammonia conversion for ammonia against reaction... Download Scientific Diagram Reaction Vessel Ammonia Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. In these conditions, some of the hydrogen and nitrogen will react to form ammonia. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The haber process consists of putting together n2 and h2. Reaction Vessel Ammonia.
From www.esru.strath.ac.uk
Ammonia storage WIND ENERGY STORAGE Reaction Vessel Ammonia The chemical reaction is given below. The reaction is reversible and the production of ammonia is. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. N 2 (g) + 3h 2 (g) → 2nh 3 (g) The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and. Reaction Vessel Ammonia.
From study.com
Synthesis of Ammonia Process & Reaction Video & Lesson Transcript Reaction Vessel Ammonia The chemical reaction is given below. In these conditions, some of the hydrogen and nitrogen will react to form ammonia. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. The reaction is reversible. Reaction Vessel Ammonia.
From ammoniaknowhow.com
Short History of Ammonia Process Past, Present and Future AmmoniaKnowHow Reaction Vessel Ammonia N 2 (g) + 3h 2 (g) → 2nh 3 (g) In these conditions, some of the hydrogen and nitrogen will react to form ammonia. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. The reaction is reversible and the production of ammonia is. The unreacted nitrogen and hydrogen, together with. Reaction Vessel Ammonia.
From www.slideserve.com
PPT Making ammonia The Haber process PowerPoint Presentation ID2054016 Reaction Vessel Ammonia The haber process consists of putting together n2 and h2 in a high. The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia.. Reaction Vessel Ammonia.
From www.tessshebaylo.com
Write The Chemical Equation Of Mixing Ammonia Nh3 With Water Tessshebaylo Reaction Vessel Ammonia The chemical reaction is given below. The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. The haber process is used. Reaction Vessel Ammonia.
From www.indiamart.com
Shakti Engineering Mild Steel Ammonia Pressure Vessels, Capacity 200 Litters at Rs 100000 in Reaction Vessel Ammonia N 2 (g) + 3h 2 (g) → 2nh 3 (g) The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. The haber process consists of putting together n2 and h2 in a high. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. In these conditions, some. Reaction Vessel Ammonia.
From www.chegg.com
Solved Nitrogen and hydrogen react to form ammonia, like Reaction Vessel Ammonia N 2 (g) + 3h 2 (g) → 2nh 3 (g) The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. The haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. A famous equilibrium reaction is the haber process for synthesizing ammonia. The chemical reaction is given below. In. Reaction Vessel Ammonia.
From www.exportersindia.com
Exporter of Spiral PP Chemical Reaction Vessel from Visakhapatnam, Andhra Pradesh by Ice North Reaction Vessel Ammonia The reaction is reversible and the production of ammonia is. In these conditions, some of the hydrogen and nitrogen will react to form ammonia. The haber process consists of putting together n2 and h2 in a high. The haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. The haber process combines nitrogen from. Reaction Vessel Ammonia.
From www.wikihow.com
3 Ways to Study the Chemical Reactions of Ammonia wikiHow Reaction Vessel Ammonia A famous equilibrium reaction is the haber process for synthesizing ammonia. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. N 2 (g) + 3h 2 (g) → 2nh 3 (g) The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. The chemical. Reaction Vessel Ammonia.
From www.numerade.com
A reaction vessel for synthesizing ammonia by reacting nitrogen and hydrogen is charged with 6. Reaction Vessel Ammonia The chemical reaction is given below. The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. The haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. A famous equilibrium reaction. Reaction Vessel Ammonia.
From maritimecyprus.com
ABS Guide to Ammonia Fueled Vessels MaritimeCyprus Reaction Vessel Ammonia A famous equilibrium reaction is the haber process for synthesizing ammonia. The haber process consists of putting together n2 and h2 in a high. The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. N 2 (g) + 3h 2 (g) → 2nh 3 (g) In these conditions, some of the hydrogen and nitrogen will react. Reaction Vessel Ammonia.
From www.indiamart.com
Krish Engineering Ammonia Pressure Vessel at Rs 220000/number in Ahmedabad ID 21111774230 Reaction Vessel Ammonia The haber process consists of putting together n2 and h2 in a high. In these conditions, some of the hydrogen and nitrogen will react to form ammonia. The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. The. Reaction Vessel Ammonia.
From www.chegg.com
Solved A reaction vessel for synthesizing ammonia by Reaction Vessel Ammonia In these conditions, some of the hydrogen and nitrogen will react to form ammonia. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. The reaction is reversible and the production of ammonia is. N 2 (g) + 3h 2 (g) → 2nh 3 (g) The chemical reaction is given below. The. Reaction Vessel Ammonia.
From vesselautomation.com
Ammonia fuel in the maritime industry Vessel Automation Reaction Vessel Ammonia Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. A famous equilibrium reaction is the haber process for synthesizing ammonia. The haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. N 2 (g) + 3h 2 (g) → 2nh 3 (g) The chemical reaction. Reaction Vessel Ammonia.
From www.chegg.com
Solved Nitrogen and hydrogen react to form ammonia, like Reaction Vessel Ammonia A famous equilibrium reaction is the haber process for synthesizing ammonia. N 2 (g) + 3h 2 (g) → 2nh 3 (g) The haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to explain the reasons for the conditions used in the process. The unreacted nitrogen and hydrogen, together with the ammonia,. Reaction Vessel Ammonia.
From www.connect-lng.com
The Ammonia Market Reaction Vessel Ammonia In these conditions, some of the hydrogen and nitrogen will react to form ammonia. The haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. The reaction is reversible and the production of ammonia is. The unreacted nitrogen and hydrogen, together with the ammonia, pass into a cooling tank. A famous equilibrium reaction is. Reaction Vessel Ammonia.