Working Electrodes Examples . Figure 35 shows examples of working electrodes from these two manufacturers. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrode reaction kinetics are affected. A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. Electrolyte solutions (low ohmic resistance): It is in direct contact with the electrolyte and plays a. Ionic solutions (nacl), molten salts, and ionic polymers (nafion). The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs.
from www.youtube.com
Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrode reaction kinetics are affected. It is in direct contact with the electrolyte and plays a. Figure 35 shows examples of working electrodes from these two manufacturers. The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. Electrolyte solutions (low ohmic resistance): Ionic solutions (nacl), molten salts, and ionic polymers (nafion). A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing.
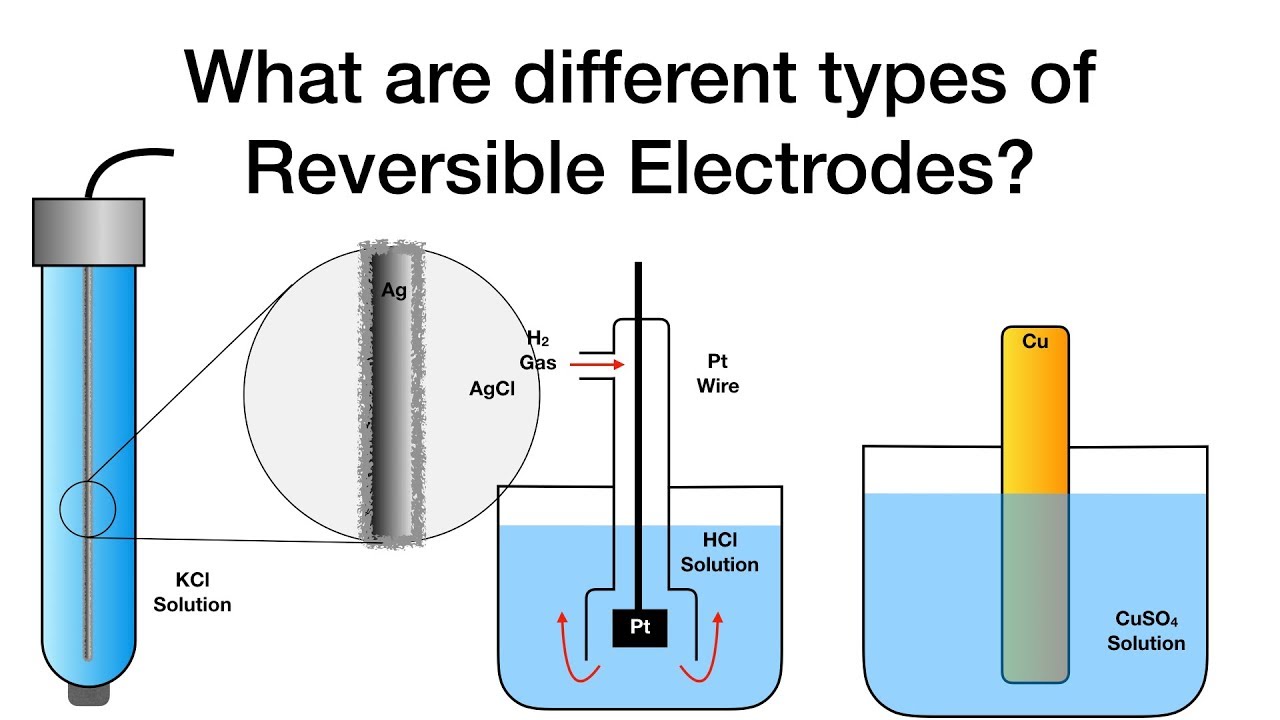
What are different types of Reversible Electrodes? Electrochemistry Physical Chemistry YouTube
Working Electrodes Examples Figure 35 shows examples of working electrodes from these two manufacturers. A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. Electrolyte solutions (low ohmic resistance): Electrode reaction kinetics are affected. Ionic solutions (nacl), molten salts, and ionic polymers (nafion). Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. Figure 35 shows examples of working electrodes from these two manufacturers. It is in direct contact with the electrolyte and plays a.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Working Electrodes Examples Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Ionic solutions (nacl), molten salts, and ionic polymers (nafion). A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable,. Working Electrodes Examples.
From exyzyaldf.blob.core.windows.net
What Can Electrodes Be Made From at Jane Crawford blog Working Electrodes Examples Figure 35 shows examples of working electrodes from these two manufacturers. The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. Electrode reaction kinetics are affected. Ionic solutions (nacl), molten salts, and ionic polymers (nafion). It is in direct contact with the electrolyte and plays a. A solid electrode—typically carbon, platinum, or gold—is. Working Electrodes Examples.
From medetronix.com
Working Electrode Holder clip type (pack of 2pcs) MTX LabsElectrochemical Devices & Accessories Working Electrodes Examples Electrode reaction kinetics are affected. The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. Ionic solutions (nacl), molten salts, and ionic polymers (nafion). Electrolyte solutions (low ohmic resistance): Figure 35 shows examples of working electrodes from these two manufacturers. Electrode reactions are a class of chemical reactions that involve the transfer of. Working Electrodes Examples.
From www.researchgate.net
Simplified 2electrode electrodeposition setup used for growing Cd1−xZnxS Download Scientific Working Electrodes Examples A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. Figure 35 shows examples of working electrodes from these two manufacturers. Electrode reaction kinetics are affected. The working electrode, also known as the active electrode, is where the. Working Electrodes Examples.
From www.researchgate.net
Schematic of the working electrode (WE), reference electrode (RE), and... Download Scientific Working Electrodes Examples Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrolyte solutions (low ohmic resistance): It is in direct contact with the electrolyte and plays a. Figure 35 shows examples of working electrodes from these two manufacturers. The working electrode, also known as the active electrode, is where the electrochemical. Working Electrodes Examples.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Working Electrodes Examples A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. Electrolyte solutions (low ohmic resistance): Electrode reaction kinetics are affected. It is in direct contact with the electrolyte and plays a. The working electrode, also known as the. Working Electrodes Examples.
From pineresearch.com
ThreeElectrode Setups Pine Research Instrumentation Store Working Electrodes Examples A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. Ionic solutions (nacl), molten salts, and ionic polymers (nafion). Figure 35 shows examples of working electrodes from these two manufacturers. Electrode reaction kinetics are affected. Electrode reactions are. Working Electrodes Examples.
From www.youtube.com
Metal Indicator Electrodes YouTube Working Electrodes Examples Ionic solutions (nacl), molten salts, and ionic polymers (nafion). Figure 35 shows examples of working electrodes from these two manufacturers. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrolyte solutions (low ohmic resistance): The working electrode, also known as the active electrode, is where the electrochemical reaction of. Working Electrodes Examples.
From www.researchgate.net
Electrolytic cell and electrodes a counter electrode; b reference... Download Scientific Diagram Working Electrodes Examples Figure 35 shows examples of working electrodes from these two manufacturers. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrode reaction kinetics are affected. It is in direct contact with the electrolyte and plays a. A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg. Working Electrodes Examples.
From chem.libretexts.org
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts Working Electrodes Examples Electrolyte solutions (low ohmic resistance): A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. Figure 35 shows examples of working electrodes from these two manufacturers. Electrode reaction kinetics are affected. Electrode reactions are a class of chemical. Working Electrodes Examples.
From www.youtube.com
IonSelective Electrode, Principle, Advantages, Disadvantages & Applications YouTube Working Electrodes Examples It is in direct contact with the electrolyte and plays a. Electrolyte solutions (low ohmic resistance): A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. Electrode reactions are a class of chemical reactions that involve the transfer. Working Electrodes Examples.
From www.researchgate.net
An electrochemical system with a working electrode (WE), a counter... Download Scientific Diagram Working Electrodes Examples A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. It is in direct contact with the electrolyte and plays a. Ionic solutions (nacl), molten salts, and ionic polymers (nafion). Electrolyte solutions (low ohmic resistance): Electrode reactions are. Working Electrodes Examples.
From www.slideserve.com
PPT ELECTROCHEMISTRY CHEM 4700 CHAPTER 4 PowerPoint Presentation, free download ID3541006 Working Electrodes Examples Electrolyte solutions (low ohmic resistance): The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at. Working Electrodes Examples.
From www.vturesource.com
Calomel Electrode Construction & Working VTU ELearning Working Electrodes Examples A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. Ionic solutions (nacl), molten salts, and ionic polymers (nafion). Electrode reaction kinetics are affected. Electrode reactions are a class of chemical reactions that involve the transfer of a. Working Electrodes Examples.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students Working Electrodes Examples Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrode reaction kinetics are affected. Ionic solutions (nacl), molten salts, and ionic polymers (nafion). It is in direct contact with the electrolyte and plays a. Figure 35 shows examples of working electrodes from these two manufacturers. Electrolyte solutions (low ohmic. Working Electrodes Examples.
From exopopjam.blob.core.windows.net
Electrode Examples at Hillary Browning blog Working Electrodes Examples It is in direct contact with the electrolyte and plays a. Electrolyte solutions (low ohmic resistance): Ionic solutions (nacl), molten salts, and ionic polymers (nafion). Figure 35 shows examples of working electrodes from these two manufacturers. The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. Electrode reaction kinetics are affected. Electrode reactions. Working Electrodes Examples.
From als-japan.com.cn
support/workingelectrode/varieties ALS 电化学 Working Electrodes Examples It is in direct contact with the electrolyte and plays a. The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. Figure 35 shows examples of working electrodes from these two manufacturers. Electrolyte solutions (low ohmic resistance): Electrode reactions are a class of chemical reactions that involve the transfer of a charged species. Working Electrodes Examples.
From slidetodoc.com
Voltammetry Chemical Modification of Working Electrodes Voltammetry Working Working Electrodes Examples The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. Ionic solutions (nacl), molten salts, and ionic polymers (nafion). Electrolyte solutions (low ohmic resistance): A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg. Working Electrodes Examples.
From www.snowhouse.ca
Working Electrodes Working Electrodes Examples A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. Electrolyte solutions (low ohmic resistance): Figure 35 shows examples of working electrodes from these two manufacturers. Ionic solutions (nacl), molten salts, and ionic polymers (nafion). The working electrode,. Working Electrodes Examples.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision Working Electrodes Examples A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. Figure 35 shows examples of working electrodes from these two manufacturers.. Working Electrodes Examples.
From pineresearch.com
TwoElectrode Setups Pine Research Instrumentation Store Working Electrodes Examples It is in direct contact with the electrolyte and plays a. The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing.. Working Electrodes Examples.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry. YouTube Working Electrodes Examples Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction. Working Electrodes Examples.
From www.researchgate.net
Photos of working electrodes designed as RDE Download Scientific Diagram Working Electrodes Examples Figure 35 shows examples of working electrodes from these two manufacturers. Ionic solutions (nacl), molten salts, and ionic polymers (nafion). A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. Electrode reaction kinetics are affected. Electrolyte solutions (low. Working Electrodes Examples.
From www.scribd.com
Working Electrode Chemistry Materials Working Electrodes Examples The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. Electrode reaction kinetics are affected. Figure 35 shows examples of working electrodes from these two manufacturers. A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 +. Working Electrodes Examples.
From semcouniversity.com
How the three electrode system works Semco University Semco university All about the Working Electrodes Examples Ionic solutions (nacl), molten salts, and ionic polymers (nafion). Figure 35 shows examples of working electrodes from these two manufacturers. Electrolyte solutions (low ohmic resistance): The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. It is in direct contact with the electrolyte and plays a. A solid electrode—typically carbon, platinum, or gold—is. Working Electrodes Examples.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free download ID592792 Working Electrodes Examples Electrolyte solutions (low ohmic resistance): The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Figure 35 shows examples of working electrodes from these two manufacturers. Ionic solutions (nacl), molten salts, and ionic. Working Electrodes Examples.
From semcouniversity.com
How the three electrode system works Semco University Semco university All about the Working Electrodes Examples Electrolyte solutions (low ohmic resistance): Ionic solutions (nacl), molten salts, and ionic polymers (nafion). A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. It is in direct contact with the electrolyte and plays a. Electrode reactions are. Working Electrodes Examples.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free download ID592792 Working Electrodes Examples Ionic solutions (nacl), molten salts, and ionic polymers (nafion). A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. It is in direct contact with the electrolyte and plays a. The working electrode, also known as the active. Working Electrodes Examples.
From chem.libretexts.org
11.4 Voltammetric and Amperometric Methods Chemistry LibreTexts Working Electrodes Examples Electrolyte solutions (low ohmic resistance): A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. Figure 35 shows examples of working electrodes from these two manufacturers. The working electrode, also known as the active electrode, is where the. Working Electrodes Examples.
From www.youtube.com
What are different types of Reversible Electrodes? Electrochemistry Physical Chemistry YouTube Working Electrodes Examples Ionic solutions (nacl), molten salts, and ionic polymers (nafion). A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. Figure 35 shows examples of working electrodes from these two manufacturers. The working electrode, also known as the active. Working Electrodes Examples.
From studylib.net
How IonSelective Electrodes Work Working Electrodes Examples Electrode reaction kinetics are affected. Electrolyte solutions (low ohmic resistance): Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is. Working Electrodes Examples.
From saylordotorg.github.io
Describing Electrochemical Cells Working Electrodes Examples Electrode reaction kinetics are affected. A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. It is in direct contact with the electrolyte and plays a. Figure 35 shows examples of working electrodes from these two manufacturers. Ionic. Working Electrodes Examples.
From pubs.rsc.org
Making electrochemistry easily accessible to the synthetic chemist Green Chemistry (RSC Working Electrodes Examples A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. It is in direct contact with the electrolyte and plays a. Figure 35 shows examples of working electrodes from these two manufacturers. Electrode reaction kinetics are affected. Ionic. Working Electrodes Examples.
From byjus.com
What is an active electrode? Explain with the help of an example Working Electrodes Examples Electrolyte solutions (low ohmic resistance): A solid electrode—typically carbon, platinum, or gold—is placed in a solution of hg 2 + and held at a potential where the reduction of hg 2 + to hg is favorable, depositing. It is in direct contact with the electrolyte and plays a. Figure 35 shows examples of working electrodes from these two manufacturers. The. Working Electrodes Examples.
From www.researchgate.net
Schematics of threeelectrode cell, standard calomel electrode as... Download Scientific Diagram Working Electrodes Examples Electrode reactions are a class of chemical reactions that involve the transfer of a charged species across an interface, most. Electrode reaction kinetics are affected. Electrolyte solutions (low ohmic resistance): It is in direct contact with the electrolyte and plays a. The working electrode, also known as the active electrode, is where the electrochemical reaction of interest occurs. Figure 35. Working Electrodes Examples.