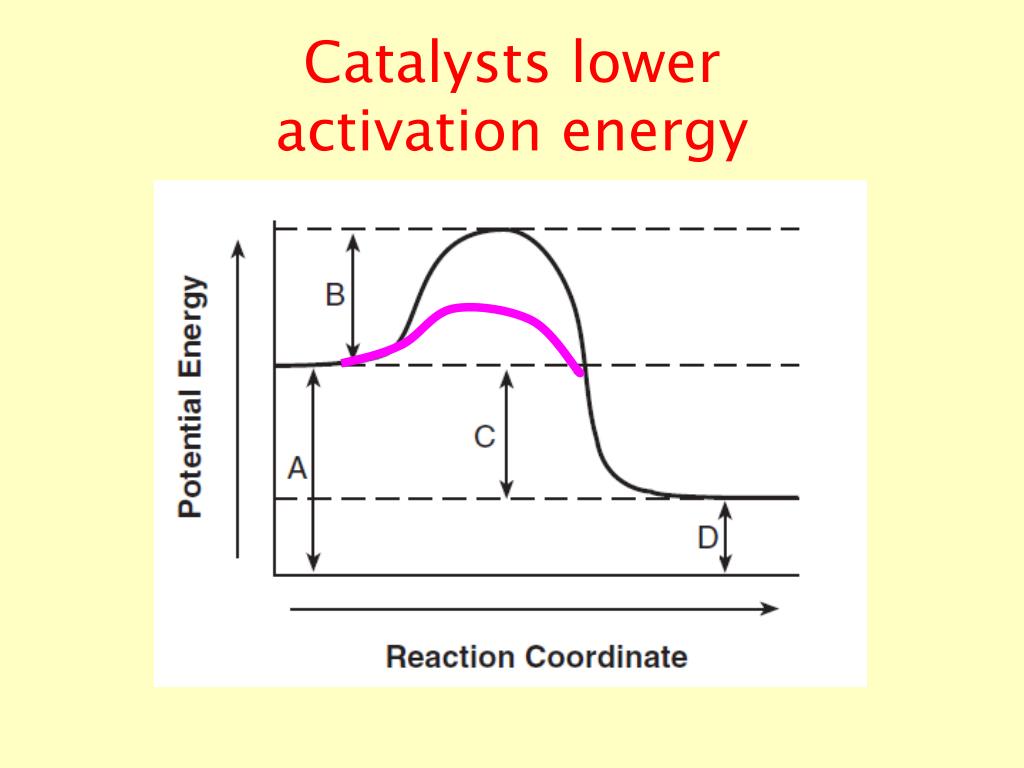
Do Catalysts Always Lower Activation Energy . catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst can open a new reaction pathway with lower activation energy. a positive catalyst lowers the activation energy of a reaction and speeds up its rate. effect of enzymes and catalysts. In contrast, a negative catalyst makes a reaction. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. estimate the activation energy for each process, and identify which one involves a catalyst. It does it by providing a partial bond which stabilises. a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction. A catalyst lowers the activation energy of a chemical reaction.
from www.slideserve.com
a positive catalyst lowers the activation energy of a reaction and speeds up its rate. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction. A catalyst lowers the activation energy of a chemical reaction. estimate the activation energy for each process, and identify which one involves a catalyst. In contrast, a negative catalyst makes a reaction. It does it by providing a partial bond which stabilises. effect of enzymes and catalysts. a catalyst can open a new reaction pathway with lower activation energy. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed.
PPT Rates of Reaction & Equilibrium PowerPoint Presentation, free
Do Catalysts Always Lower Activation Energy A catalyst lowers the activation energy of a chemical reaction. a positive catalyst lowers the activation energy of a reaction and speeds up its rate. effect of enzymes and catalysts. estimate the activation energy for each process, and identify which one involves a catalyst. a catalyst can open a new reaction pathway with lower activation energy. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It does it by providing a partial bond which stabilises. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. In contrast, a negative catalyst makes a reaction. a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction. A catalyst lowers the activation energy of a chemical reaction.
From slideplayer.com
Chemical Honors Unit ppt download Do Catalysts Always Lower Activation Energy In contrast, a negative catalyst makes a reaction. a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction. a catalyst can open a new reaction pathway with lower activation energy. catalysts are defined as substances that participate in a chemical reaction but are. Do Catalysts Always Lower Activation Energy.
From www.chim.lu
Activation energy and catalysis Do Catalysts Always Lower Activation Energy estimate the activation energy for each process, and identify which one involves a catalyst. effect of enzymes and catalysts. A catalyst lowers the activation energy of a chemical reaction. a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction. a positive catalyst. Do Catalysts Always Lower Activation Energy.
From exyfvrfps.blob.core.windows.net
Do All Catalysts Lower Activation Energy at Shirley Tolliver blog Do Catalysts Always Lower Activation Energy A catalyst lowers the activation energy of a chemical reaction. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. It does it by providing a partial bond which stabilises. estimate the activation energy for each process, and identify which one involves a catalyst. a catalyst provides an alternative route. Do Catalysts Always Lower Activation Energy.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Do Catalysts Always Lower Activation Energy effect of enzymes and catalysts. A catalyst lowers the activation energy of a chemical reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. estimate the activation energy for each process, and identify which one involves a catalyst. a catalyst can open a new reaction pathway with. Do Catalysts Always Lower Activation Energy.
From www.slideserve.com
PPT Enzymes Biological Catalysts PowerPoint Presentation ID5736986 Do Catalysts Always Lower Activation Energy a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. effect of enzymes and catalysts. a catalyst can open a new reaction pathway with. Do Catalysts Always Lower Activation Energy.
From www.slideserve.com
PPT Catalysis PowerPoint Presentation, free download ID2842456 Do Catalysts Always Lower Activation Energy effect of enzymes and catalysts. a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction. In contrast, a negative catalyst makes a reaction. a positive catalyst lowers the activation energy of a reaction and speeds up its rate. a catalyst can open. Do Catalysts Always Lower Activation Energy.
From www.chemistrylearner.com
Activation Energy Definition, Formula, and Graph Do Catalysts Always Lower Activation Energy a catalyst can open a new reaction pathway with lower activation energy. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It does it by providing a partial bond which stabilises. estimate the activation energy for each process, and identify which one involves a catalyst. A catalyst lowers. Do Catalysts Always Lower Activation Energy.
From www.slideserve.com
PPT Reaction Rate and Equilibrium PowerPoint Presentation, free Do Catalysts Always Lower Activation Energy a catalyst can open a new reaction pathway with lower activation energy. estimate the activation energy for each process, and identify which one involves a catalyst. A catalyst lowers the activation energy of a chemical reaction. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. a positive catalyst. Do Catalysts Always Lower Activation Energy.
From slideplayer.com
Chemical Unit 10. the study of the speeds Do Catalysts Always Lower Activation Energy catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. a catalyst can open a new reaction pathway with lower activation energy. a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction. catalysts affect the. Do Catalysts Always Lower Activation Energy.
From www.slideserve.com
PPT Chapter 5 Enzymes PowerPoint Presentation, free download ID Do Catalysts Always Lower Activation Energy effect of enzymes and catalysts. It does it by providing a partial bond which stabilises. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction.. Do Catalysts Always Lower Activation Energy.
From vdocuments.mx
Catalysts Catalystlowers activation energy of a chemical reaction and Do Catalysts Always Lower Activation Energy catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. a catalyst can open a new reaction pathway with lower activation energy. In contrast, a negative catalyst makes a reaction. It does. Do Catalysts Always Lower Activation Energy.
From slideplayer.com
& Equilibrium ppt download Do Catalysts Always Lower Activation Energy a catalyst can open a new reaction pathway with lower activation energy. estimate the activation energy for each process, and identify which one involves a catalyst. A catalyst lowers the activation energy of a chemical reaction. It does it by providing a partial bond which stabilises. catalysts are defined as substances that participate in a chemical reaction. Do Catalysts Always Lower Activation Energy.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology Do Catalysts Always Lower Activation Energy estimate the activation energy for each process, and identify which one involves a catalyst. a positive catalyst lowers the activation energy of a reaction and speeds up its rate. a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction. A catalyst lowers the. Do Catalysts Always Lower Activation Energy.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) Do Catalysts Always Lower Activation Energy a positive catalyst lowers the activation energy of a reaction and speeds up its rate. A catalyst lowers the activation energy of a chemical reaction. effect of enzymes and catalysts. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. estimate the activation energy for each process, and. Do Catalysts Always Lower Activation Energy.
From www.researchgate.net
Free energy of activation of uncatalyzed and catalyzed reactions Do Catalysts Always Lower Activation Energy a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction. In contrast, a negative catalyst makes a reaction. a positive catalyst lowers the activation energy of a reaction and speeds up its rate. A catalyst lowers the activation energy of a chemical reaction. . Do Catalysts Always Lower Activation Energy.
From slideplayer.com
Enzymes. ppt download Do Catalysts Always Lower Activation Energy estimate the activation energy for each process, and identify which one involves a catalyst. effect of enzymes and catalysts. a positive catalyst lowers the activation energy of a reaction and speeds up its rate. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst can. Do Catalysts Always Lower Activation Energy.
From www.slideserve.com
PPT Enzyme Biological Catalyst (Part ii) PowerPoint Presentation Do Catalysts Always Lower Activation Energy In contrast, a negative catalyst makes a reaction. effect of enzymes and catalysts. estimate the activation energy for each process, and identify which one involves a catalyst. a catalyst can open a new reaction pathway with lower activation energy. A catalyst lowers the activation energy of a chemical reaction. catalysts are defined as substances that participate. Do Catalysts Always Lower Activation Energy.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free Do Catalysts Always Lower Activation Energy In contrast, a negative catalyst makes a reaction. a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction. A catalyst lowers the activation energy of a chemical reaction. catalysts are defined as substances that participate in a chemical reaction but are not changed or. Do Catalysts Always Lower Activation Energy.
From 2012books.lardbucket.org
Catalysis Do Catalysts Always Lower Activation Energy a catalyst can open a new reaction pathway with lower activation energy. It does it by providing a partial bond which stabilises. effect of enzymes and catalysts. In contrast, a negative catalyst makes a reaction. estimate the activation energy for each process, and identify which one involves a catalyst. a catalyst provides an alternative route for. Do Catalysts Always Lower Activation Energy.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited Do Catalysts Always Lower Activation Energy a positive catalyst lowers the activation energy of a reaction and speeds up its rate. a catalyst can open a new reaction pathway with lower activation energy. A catalyst lowers the activation energy of a chemical reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. effect. Do Catalysts Always Lower Activation Energy.
From btccasting.weebly.com
Enzymes Lower The Activation Energy Of A Reaction btccasting Do Catalysts Always Lower Activation Energy catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction. It does it by providing a partial bond which stabilises. catalysts affect the rate of a. Do Catalysts Always Lower Activation Energy.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Do Catalysts Always Lower Activation Energy a positive catalyst lowers the activation energy of a reaction and speeds up its rate. In contrast, a negative catalyst makes a reaction. It does it by providing a partial bond which stabilises. estimate the activation energy for each process, and identify which one involves a catalyst. effect of enzymes and catalysts. A catalyst lowers the activation. Do Catalysts Always Lower Activation Energy.
From slideplayer.com
Enzymes. ppt download Do Catalysts Always Lower Activation Energy catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a positive catalyst lowers the activation energy of a reaction and speeds up its rate. a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction.. Do Catalysts Always Lower Activation Energy.
From www.slideserve.com
PPT Rates of Reaction & Equilibrium PowerPoint Presentation, free Do Catalysts Always Lower Activation Energy estimate the activation energy for each process, and identify which one involves a catalyst. In contrast, a negative catalyst makes a reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a catalyst provides an alternative route for the reaction with a lower activation energy. it does not. Do Catalysts Always Lower Activation Energy.
From slideplayer.com
Chapter 12 Chemical ppt download Do Catalysts Always Lower Activation Energy It does it by providing a partial bond which stabilises. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. a positive catalyst lowers the activation energy of a reaction and speeds. Do Catalysts Always Lower Activation Energy.
From hxebmwake.blob.core.windows.net
A Catalyst Lowers The Activation Energy But Does Not Affect The Do Catalysts Always Lower Activation Energy estimate the activation energy for each process, and identify which one involves a catalyst. a catalyst provides an alternative route for the reaction with a lower activation energy. it does not lower the activation energy of the reaction. a catalyst can open a new reaction pathway with lower activation energy. catalysts are defined as substances that. Do Catalysts Always Lower Activation Energy.
From byjus.com
Activation Energy Do Catalysts Always Lower Activation Energy In contrast, a negative catalyst makes a reaction. effect of enzymes and catalysts. a positive catalyst lowers the activation energy of a reaction and speeds up its rate. a catalyst can open a new reaction pathway with lower activation energy. It does it by providing a partial bond which stabilises. catalysts affect the rate of a. Do Catalysts Always Lower Activation Energy.
From exyfvrfps.blob.core.windows.net
Do All Catalysts Lower Activation Energy at Shirley Tolliver blog Do Catalysts Always Lower Activation Energy A catalyst lowers the activation energy of a chemical reaction. a catalyst can open a new reaction pathway with lower activation energy. effect of enzymes and catalysts. It does it by providing a partial bond which stabilises. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. In contrast,. Do Catalysts Always Lower Activation Energy.
From slideplayer.com
Chemical Reactions. ppt download Do Catalysts Always Lower Activation Energy catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. In contrast, a negative catalyst makes a reaction. effect of enzymes and catalysts. a catalyst can open a new reaction pathway with lower activation energy. a positive catalyst lowers the activation energy of a reaction and speeds up. Do Catalysts Always Lower Activation Energy.
From ar.inspiredpencil.com
Activation Energy Catalyst Do Catalysts Always Lower Activation Energy effect of enzymes and catalysts. A catalyst lowers the activation energy of a chemical reaction. catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. a catalyst provides an alternative route. Do Catalysts Always Lower Activation Energy.
From www.slideserve.com
PPT RATES OF REACTION A guide for GCSE students PowerPoint Do Catalysts Always Lower Activation Energy catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It does it by providing a partial bond which stabilises. a positive catalyst lowers the activation energy of a reaction and speeds up its rate. estimate the activation energy for each process, and identify which one involves a catalyst.. Do Catalysts Always Lower Activation Energy.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2568751 Do Catalysts Always Lower Activation Energy In contrast, a negative catalyst makes a reaction. effect of enzymes and catalysts. A catalyst lowers the activation energy of a chemical reaction. catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. estimate the activation energy for each process, and identify which one involves a catalyst. It does it. Do Catalysts Always Lower Activation Energy.
From kenya-khurst.blogspot.com
Catalysts Lower the Activation Energy of a Reaction by Do Catalysts Always Lower Activation Energy estimate the activation energy for each process, and identify which one involves a catalyst. A catalyst lowers the activation energy of a chemical reaction. a positive catalyst lowers the activation energy of a reaction and speeds up its rate. a catalyst can open a new reaction pathway with lower activation energy. catalysts are defined as substances. Do Catalysts Always Lower Activation Energy.
From www.slideserve.com
PPT Reaction PowerPoint Presentation, free download ID1835084 Do Catalysts Always Lower Activation Energy catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. a positive catalyst lowers the activation energy of a reaction and speeds up its rate. In contrast, a negative catalyst makes a reaction. It does it by providing a partial bond which stabilises. effect of enzymes and catalysts. a. Do Catalysts Always Lower Activation Energy.
From byjus.com
Activation Energy Definition, Formula, SI Units, Examples, Calculation Do Catalysts Always Lower Activation Energy catalysts are defined as substances that participate in a chemical reaction but are not changed or consumed. A catalyst lowers the activation energy of a chemical reaction. estimate the activation energy for each process, and identify which one involves a catalyst. It does it by providing a partial bond which stabilises. catalysts affect the rate of a. Do Catalysts Always Lower Activation Energy.