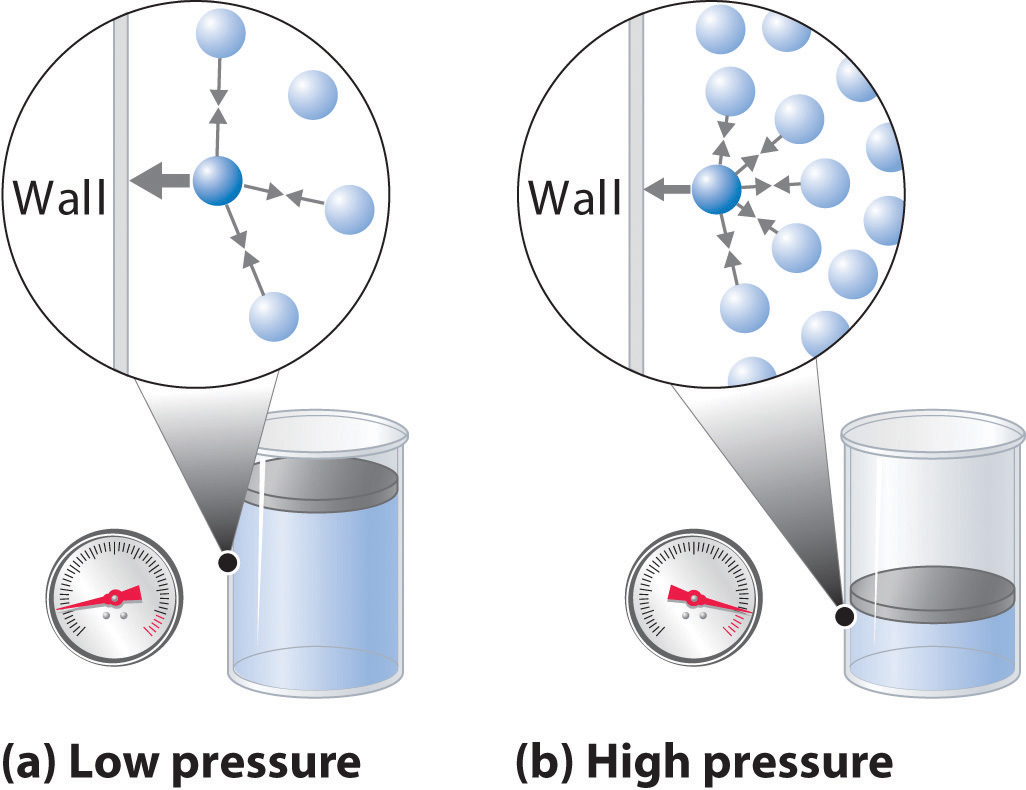
Lighter Gas Pressure . pressure of gas mixtures. the main task of vacuum technology is to reduce the particle number density n inside a given volume v. Examine kinetic energy and speed. the ideal gas constant is given the symbol r, and is equal to 0.08206 atm!l/mol!k. Postulate 3 of the kinetic molecular theory of gases states that gas molecules exert no attractive or repulsive. In this experiment, you will determine the value. In the kelvin scale, \(0 \:. Since the temperatures are the same, both gases. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or volume changes. lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. assuming the two gases are ideal, both are therefore at the same pressure.
from www.askiitians.com
the main task of vacuum technology is to reduce the particle number density n inside a given volume v. Since the temperatures are the same, both gases. Examine kinetic energy and speed. pressure of gas mixtures. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. In this experiment, you will determine the value. In the kelvin scale, \(0 \:. assuming the two gases are ideal, both are therefore at the same pressure. the ideal gas constant is given the symbol r, and is equal to 0.08206 atm!l/mol!k.
Gas Laws And Properties Of Gases Study Material for IIT JEE askIITians
Lighter Gas Pressure the main task of vacuum technology is to reduce the particle number density n inside a given volume v. Examine kinetic energy and speed. Postulate 3 of the kinetic molecular theory of gases states that gas molecules exert no attractive or repulsive. pressure of gas mixtures. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. assuming the two gases are ideal, both are therefore at the same pressure. the main task of vacuum technology is to reduce the particle number density n inside a given volume v. Since the temperatures are the same, both gases. lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or volume changes. In the kelvin scale, \(0 \:. In this experiment, you will determine the value. the ideal gas constant is given the symbol r, and is equal to 0.08206 atm!l/mol!k.
From www.youtube.com
How to fix lighter low pressure problem, Wonder gas lighter ko repair Lighter Gas Pressure assuming the two gases are ideal, both are therefore at the same pressure. Postulate 3 of the kinetic molecular theory of gases states that gas molecules exert no attractive or repulsive. Since the temperatures are the same, both gases. In this experiment, you will determine the value. this ideal gas law calculator will help you establish the properties. Lighter Gas Pressure.
From www.dreamstime.com
How Does a Lighter Work? stock vector. Illustration of fuel 257316938 Lighter Gas Pressure the ideal gas constant is given the symbol r, and is equal to 0.08206 atm!l/mol!k. pressure of gas mixtures. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. In the kelvin. Lighter Gas Pressure.
From www.youtube.com
How to Make a gas Lighters YouTube Lighter Gas Pressure the ideal gas constant is given the symbol r, and is equal to 0.08206 atm!l/mol!k. Examine kinetic energy and speed. assuming the two gases are ideal, both are therefore at the same pressure. In the kelvin scale, \(0 \:. lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. Since the temperatures. Lighter Gas Pressure.
From www.backdoorsurvival.com
10 Types of Lighters You Should Know About in 2024 Easy to Use Lighter Gas Pressure Postulate 3 of the kinetic molecular theory of gases states that gas molecules exert no attractive or repulsive. pressure of gas mixtures. Since the temperatures are the same, both gases. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the ideal gas constant is given the symbol. Lighter Gas Pressure.
From lumarko.co.uk
Leifheit Gas Lighter Pro Line Inox 21130 Other PRO LINE INOX 21130 Lighter Gas Pressure assuming the two gases are ideal, both are therefore at the same pressure. the main task of vacuum technology is to reduce the particle number density n inside a given volume v. Postulate 3 of the kinetic molecular theory of gases states that gas molecules exert no attractive or repulsive. lighter gases will have higher velocities than. Lighter Gas Pressure.
From www.youtube.com
How to refill a lighter with log Gas refilling the electronic lighter Lighter Gas Pressure this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or volume changes. In this experiment, you will determine the value. Postulate 3 of the kinetic molecular theory of gases states that gas molecules exert no attractive or repulsive. measure the temperature and pressure, and discover how the properties. Lighter Gas Pressure.
From www.youtube.com
What's Inside GASLIGHTER YouTube Lighter Gas Pressure the ideal gas constant is given the symbol r, and is equal to 0.08206 atm!l/mol!k. pressure of gas mixtures. assuming the two gases are ideal, both are therefore at the same pressure. In this experiment, you will determine the value. measure the temperature and pressure, and discover how the properties of the gas vary in relation. Lighter Gas Pressure.
From www.youtube.com
What is Inside a Gas Lighter Components & Working Explained YouTube Lighter Gas Pressure the main task of vacuum technology is to reduce the particle number density n inside a given volume v. In this experiment, you will determine the value. Postulate 3 of the kinetic molecular theory of gases states that gas molecules exert no attractive or repulsive. lighter gases will have higher velocities than heavier gases, at the same temperature. Lighter Gas Pressure.
From www.youtube.com
What's Inside a Gaslighter YouTube Lighter Gas Pressure assuming the two gases are ideal, both are therefore at the same pressure. In the kelvin scale, \(0 \:. In this experiment, you will determine the value. pressure of gas mixtures. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Since the temperatures are the same, both. Lighter Gas Pressure.
From byjus.com
Partial Pressure Formula, Dalton's Law, Mixture of Ideal Gas, Examples Lighter Gas Pressure In the kelvin scale, \(0 \:. the main task of vacuum technology is to reduce the particle number density n inside a given volume v. pressure of gas mixtures. the ideal gas constant is given the symbol r, and is equal to 0.08206 atm!l/mol!k. In this experiment, you will determine the value. lighter gases will have. Lighter Gas Pressure.
From www.youtube.com
How To Turn A Gas Lighter into a practical refillable lighter YouTube Lighter Gas Pressure lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. Examine kinetic energy and speed. Since the temperatures are the same, both gases. In this experiment, you will determine the value. assuming the two gases are ideal, both are therefore at the same pressure. measure the temperature and pressure, and discover how. Lighter Gas Pressure.
From www.scienceabc.com
How Does A Lighter Work? Lighter Gas Pressure this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or volume changes. the main task of vacuum technology is to reduce the particle number density n inside a given volume v. Examine kinetic energy and speed. the ideal gas constant is given the symbol r, and is. Lighter Gas Pressure.
From love-lighters.com
How does cigarette lighter gas refill? and What do you use love.lighters Lighter Gas Pressure In this experiment, you will determine the value. lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or volume changes. In the kelvin scale, \(0 \:. the ideal gas constant is given. Lighter Gas Pressure.
From www.youtube.com
Relationship between pressure and temperature (derivation, Lighter Gas Pressure Postulate 3 of the kinetic molecular theory of gases states that gas molecules exert no attractive or repulsive. Examine kinetic energy and speed. lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. the main task of vacuum technology is to reduce the particle number density n inside a given volume v. . Lighter Gas Pressure.
From fsevergreentree.en.made-in-china.com
Customized Logo High Pressure Refill Lighter Gas Canister 270g China Lighter Gas Pressure Since the temperatures are the same, both gases. pressure of gas mixtures. In this experiment, you will determine the value. Postulate 3 of the kinetic molecular theory of gases states that gas molecules exert no attractive or repulsive. In the kelvin scale, \(0 \:. Examine kinetic energy and speed. assuming the two gases are ideal, both are therefore. Lighter Gas Pressure.
From www.youtube.com
How piezo crystal Gas Lighter works? How to repair Gas Lighter? Gas Lighter Gas Pressure Examine kinetic energy and speed. pressure of gas mixtures. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the ideal gas constant is given the symbol r, and is equal to 0.08206 atm!l/mol!k. this ideal gas law calculator will help you establish the properties of an. Lighter Gas Pressure.
From www.youtube.com
Gas lighter how to refill lighter utilities aim and flame bbq Lighter Gas Pressure Postulate 3 of the kinetic molecular theory of gases states that gas molecules exert no attractive or repulsive. In this experiment, you will determine the value. the ideal gas constant is given the symbol r, and is equal to 0.08206 atm!l/mol!k. pressure of gas mixtures. Since the temperatures are the same, both gases. this ideal gas law. Lighter Gas Pressure.
From www.askiitians.com
Gas Laws And Properties Of Gases Study Material for IIT JEE askIITians Lighter Gas Pressure Since the temperatures are the same, both gases. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. pressure of gas mixtures. the main task of vacuum technology is to reduce the particle number density n inside a given volume v. lighter gases will have higher velocities. Lighter Gas Pressure.
From www.youtube.com
How To Light A Gas Stove Using A Lighter YouTube Lighter Gas Pressure lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. pressure of gas mixtures. In the kelvin scale, \(0 \:. the ideal gas constant is given the symbol r, and is equal. Lighter Gas Pressure.
From www.closeracingsupply.com
QRP61719 Fuel Pressure Warning Light Kit Lighter Gas Pressure lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the main task of vacuum technology is to reduce the particle number density n inside a given volume v. In this experiment, you. Lighter Gas Pressure.
From www.amazon.com
Ultra Pure Plus 420ml Butane British European Refined Lighter Gas Pressure the ideal gas constant is given the symbol r, and is equal to 0.08206 atm!l/mol!k. In the kelvin scale, \(0 \:. Since the temperatures are the same, both gases. pressure of gas mixtures. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. this ideal gas law. Lighter Gas Pressure.
From www.amazon.in
DipDark Refilable Gas Lighter for Kitchen Gas Stove with Refiller Gas Lighter Gas Pressure Since the temperatures are the same, both gases. lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the ideal gas constant is given the symbol r, and is equal to 0.08206 atm!l/mol!k.. Lighter Gas Pressure.
From www.youtube.com
Measuring & setting Gas Pressure using a manometer YouTube Lighter Gas Pressure Postulate 3 of the kinetic molecular theory of gases states that gas molecules exert no attractive or repulsive. the main task of vacuum technology is to reduce the particle number density n inside a given volume v. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. this. Lighter Gas Pressure.
From www.neptunecigar.com
How to refill your lighter Lighter Gas Pressure In this experiment, you will determine the value. assuming the two gases are ideal, both are therefore at the same pressure. Postulate 3 of the kinetic molecular theory of gases states that gas molecules exert no attractive or repulsive. Examine kinetic energy and speed. measure the temperature and pressure, and discover how the properties of the gas vary. Lighter Gas Pressure.
From www.achasoda.com
High Output Fire Cigarette Lighter Machine With Full Pneumatic Control Lighter Gas Pressure the ideal gas constant is given the symbol r, and is equal to 0.08206 atm!l/mol!k. the main task of vacuum technology is to reduce the particle number density n inside a given volume v. Examine kinetic energy and speed. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each. Lighter Gas Pressure.
From lightertorch.com
Voltage in a Gas Lighter Lighter Torch Lighter Gas Pressure pressure of gas mixtures. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. In the kelvin scale, \(0 \:. Since the temperatures are the same, both gases. lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. this ideal gas law. Lighter Gas Pressure.
From enginelibraryzimman.z4.web.core.windows.net
Gas Lighter Circuit Diagram Lighter Gas Pressure assuming the two gases are ideal, both are therefore at the same pressure. the ideal gas constant is given the symbol r, and is equal to 0.08206 atm!l/mol!k. pressure of gas mixtures. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the main task of. Lighter Gas Pressure.
From www.youtube.com
Super Speed Natural sui gas compressor review l How to increase gas Lighter Gas Pressure assuming the two gases are ideal, both are therefore at the same pressure. Examine kinetic energy and speed. In the kelvin scale, \(0 \:. Postulate 3 of the kinetic molecular theory of gases states that gas molecules exert no attractive or repulsive. In this experiment, you will determine the value. the main task of vacuum technology is to. Lighter Gas Pressure.
From www.youtube.com
How to refill lighter with gas? How to refill gas lighter with Butane Lighter Gas Pressure lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. assuming the two gases are ideal, both are therefore at the same pressure. In the kelvin scale, \(0 \:. pressure of gas mixtures. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each. Lighter Gas Pressure.
From www.youtube.com
How to refill a lighter with fluid gas. YouTube Lighter Gas Pressure Since the temperatures are the same, both gases. lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. assuming the two gases are ideal, both are therefore at the same pressure. Examine kinetic energy and speed. this ideal gas law calculator will help you establish the properties of an ideal gas subject. Lighter Gas Pressure.
From blinkit.com
Gas Pressure Lighter Ghost Print Bongchie Price Buy Online at ₹40 Lighter Gas Pressure this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or volume changes. In this experiment, you will determine the value. assuming the two gases are ideal, both are therefore at the same pressure. pressure of gas mixtures. lighter gases will have higher velocities than heavier gases,. Lighter Gas Pressure.
From www.youtube.com
What is inside a gas stove lighter, how does work a gas stove lighter Lighter Gas Pressure In this experiment, you will determine the value. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure, temperature, or volume changes. the main task of vacuum technology is to. Lighter Gas Pressure.
From www.labxyi.com
Lighter gas storage tank pressure tester Lighter Gas Pressure pressure of gas mixtures. lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. the main task of vacuum technology is to reduce the particle number density n inside a given volume v. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each. Lighter Gas Pressure.
From www.indiamart.com
2157 Stainless Steel Electronic Gas Lighter for lighting Gas Stove at Lighter Gas Pressure In this experiment, you will determine the value. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the main task of vacuum technology is to reduce the particle number density n inside a given volume v. Since the temperatures are the same, both gases. the ideal gas. Lighter Gas Pressure.
From www.radiantbong.com
WholesaleMonsoon Lighter Gas Retro Grinding Wheel Lighter Cigarette Lighter Gas Pressure the main task of vacuum technology is to reduce the particle number density n inside a given volume v. lighter gases will have higher velocities than heavier gases, at the same temperature and pressure. Examine kinetic energy and speed. this ideal gas law calculator will help you establish the properties of an ideal gas subject to pressure,. Lighter Gas Pressure.