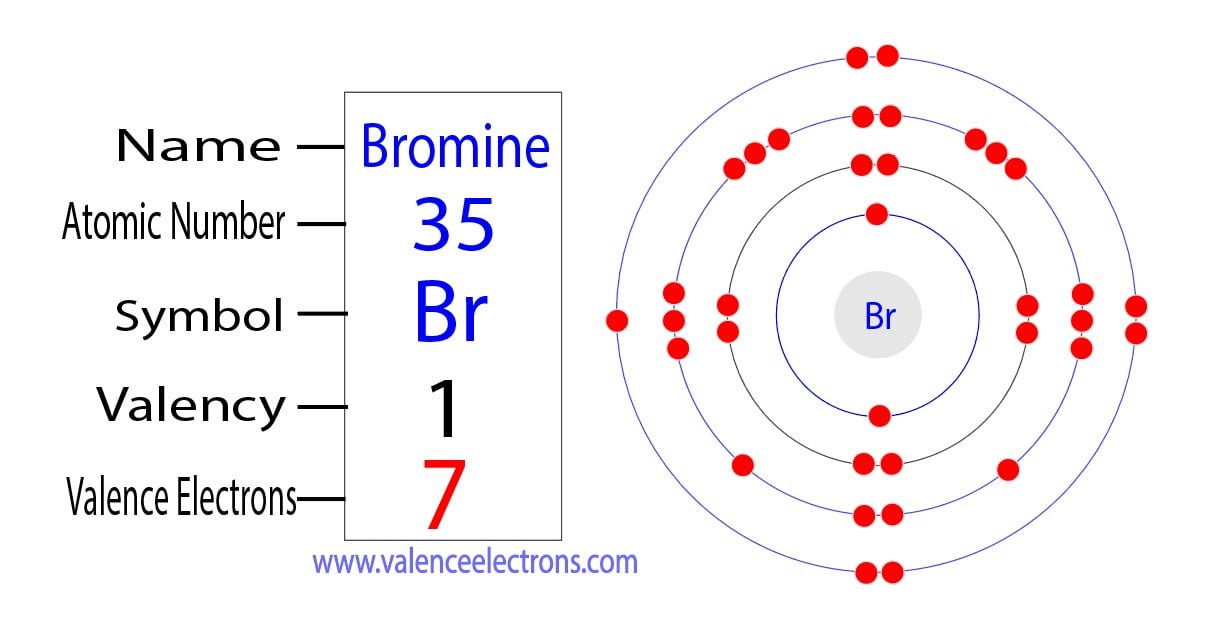
Bromine Atom Unpaired Electrons . B) write the electron configuration. Find out the molecular geometry, hybridisation,. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. One lone pair and three unpaired electrons. Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone pairs on each atom. A) determine the total number of unpaired electrons. C) identify the core electrons and the. Group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: Based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35. Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons.
from valenceelectrons.com
Find out the molecular geometry, hybridisation,. One lone pair and three unpaired electrons. Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone pairs on each atom. A) determine the total number of unpaired electrons. Based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. B) write the electron configuration. Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35. Group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol:
How Many Valence Electrons Does Bromine (Br) Have?
Bromine Atom Unpaired Electrons One lone pair and three unpaired electrons. Based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. B) write the electron configuration. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. Find out the molecular geometry, hybridisation,. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. Group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: One lone pair and three unpaired electrons. C) identify the core electrons and the. Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone pairs on each atom. A) determine the total number of unpaired electrons. Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35.
From valenceelectrons.com
How Many Valence Electrons Does Bromine (Br) Have? Bromine Atom Unpaired Electrons Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. Find out the molecular geometry, hybridisation,. Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. A) determine. Bromine Atom Unpaired Electrons.
From app.emaze.com
Bromine ) on emaze Bromine Atom Unpaired Electrons Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. Based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. Learn how to draw the lewis structure of bromine, a diatomic molecule with 14. Bromine Atom Unpaired Electrons.
From www.sciencephoto.com
Bromine, atomic structure Stock Image C018/3716 Science Photo Library Bromine Atom Unpaired Electrons Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. A) determine the total number of unpaired electrons. Find out the molecular geometry, hybridisation,. One lone pair and three unpaired electrons. Group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: Find out its natural and unstable isotopes, electron. Bromine Atom Unpaired Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Atom Unpaired Electrons Find out the molecular geometry, hybridisation,. Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35. Group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone. Bromine Atom Unpaired Electrons.
From www.alamy.com
3d render of atom structure of bromine isolated over white background Bromine Atom Unpaired Electrons Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. B) write the electron configuration. One lone pair and three unpaired electrons. C) identify the. Bromine Atom Unpaired Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Unpaired Electrons Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35. Based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. Learn how to draw the lewis structure. Bromine Atom Unpaired Electrons.
From slideplayer.com
Alkanes, Alkenes and Halogenoalkanes ppt download Bromine Atom Unpaired Electrons B) write the electron configuration. Find out the molecular geometry, hybridisation,. Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. One lone pair and three unpaired electrons. Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35. Based on the structures shown above,. Bromine Atom Unpaired Electrons.
From www.dreamstime.com
Atom of Bromine with Detailed Core and Its 35 Electrons on Black Stock Bromine Atom Unpaired Electrons Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. C) identify the core electrons and the. Based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. B) write the electron configuration. Group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol:. Bromine Atom Unpaired Electrons.
From slideplayer.com
Alkanes, Alkenes and Halogenoalkanes ppt download Bromine Atom Unpaired Electrons Based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. B) write the electron configuration. Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone pairs on each atom. Find out the molecular geometry, hybridisation,. Learn how to draw the lewis structure of bromine,. Bromine Atom Unpaired Electrons.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Bromine Atom Unpaired Electrons Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35. A) determine the total number of unpaired electrons. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. One lone pair. Bromine Atom Unpaired Electrons.
From autoctrls.com
Understanding the Bromine Electron Dot Diagram A Comprehensive Guide Bromine Atom Unpaired Electrons Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. B) write the electron configuration. A) determine the total number of unpaired electrons. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. Group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: Based on the. Bromine Atom Unpaired Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Atom Unpaired Electrons B) write the electron configuration. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. A) determine the total number of unpaired electrons. Based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. Group. Bromine Atom Unpaired Electrons.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Atom Unpaired Electrons A) determine the total number of unpaired electrons. C) identify the core electrons and the. Based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence. Bromine Atom Unpaired Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Unpaired Electrons A) determine the total number of unpaired electrons. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. Find out the molecular geometry, hybridisation,. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. Group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: Learn how. Bromine Atom Unpaired Electrons.
From www.alamy.com
Symbol and electron diagram for Bromine illustration Stock Vector Image Bromine Atom Unpaired Electrons A) determine the total number of unpaired electrons. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35.. Bromine Atom Unpaired Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Bromine Atom Unpaired Electrons Based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. C) identify the core electrons and the. Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. A) determine the total number of unpaired electrons. Learn about the number and arrangement of protons, neutrons and electrons in. Bromine Atom Unpaired Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Atom Unpaired Electrons C) identify the core electrons and the. Group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35. Find out the molecular geometry, hybridisation,. Learn how to draw the lewis structure of bromine, a diatomic molecule. Bromine Atom Unpaired Electrons.
From hxezlfuiq.blob.core.windows.net
Bromine Unpaired Electrons at Roy Aguilar blog Bromine Atom Unpaired Electrons One lone pair and three unpaired electrons. C) identify the core electrons and the. A) determine the total number of unpaired electrons. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone pairs on each atom. Learn the. Bromine Atom Unpaired Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Atom Unpaired Electrons Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. Find out the molecular geometry, hybridisation,. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. B) write the electron configuration. Learn about the number. Bromine Atom Unpaired Electrons.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Atom Unpaired Electrons Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone pairs on each atom. Based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. Learn the difference between paramagnetism and diamagnetism,. Bromine Atom Unpaired Electrons.
From www.dreamstime.com
Bromine Atom, with Mass and Energy Levels. Stock Vector Illustration Bromine Atom Unpaired Electrons Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone pairs on each atom. A) determine the total number of unpaired electrons. B) write the electron configuration. Group 15 elements such as nitrogen have five. Bromine Atom Unpaired Electrons.
From slideplayer.com
Chemical Bonding I CHEMISTRY 161 Chapter 9 Chemical Bonding I ppt Bromine Atom Unpaired Electrons Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. C) identify the core electrons and the. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone pairs on each atom. One. Bromine Atom Unpaired Electrons.
From hxezlfuiq.blob.core.windows.net
Bromine Unpaired Electrons at Roy Aguilar blog Bromine Atom Unpaired Electrons A) determine the total number of unpaired electrons. Find out the molecular geometry, hybridisation,. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. C) identify the core electrons and the. Group 15 elements such as nitrogen have five valence electrons. Bromine Atom Unpaired Electrons.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Atom Unpaired Electrons One lone pair and three unpaired electrons. Group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: Find out the molecular geometry, hybridisation,. C) identify the core electrons and the. A) determine the total number of unpaired electrons. Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with. Bromine Atom Unpaired Electrons.
From www.alamy.com
Symbol and electron diagram for Bromine illustration Stock Vector Image Bromine Atom Unpaired Electrons B) write the electron configuration. Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35. Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. A) determine the total number of unpaired electrons. Based on the structures shown above, bromine contains 1 unpaired electron,. Bromine Atom Unpaired Electrons.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Atom Unpaired Electrons Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35. Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. Based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. A) determine the total number of unpaired. Bromine Atom Unpaired Electrons.
From www.numerade.com
SOLVED For the Bromine atom a) Determine the total number of unpaired Bromine Atom Unpaired Electrons A) determine the total number of unpaired electrons. Group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: B) write the electron configuration. C) identify the core electrons and the. Find out the molecular geometry, hybridisation,. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. Learn how to draw the lewis. Bromine Atom Unpaired Electrons.
From www.numerade.com
SOLVEDWrite orbital diagrams for the valence electrons and indicate Bromine Atom Unpaired Electrons Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone pairs on each atom. Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. Group 15 elements such. Bromine Atom Unpaired Electrons.
From www.doubtnut.com
Total no of unpaired electrons in bromine atom is x, atomic number of Bromine Atom Unpaired Electrons Find out the molecular geometry, hybridisation,. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. B) write the electron configuration. Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone pairs on each atom. One lone pair and three unpaired electrons. Find out its. Bromine Atom Unpaired Electrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Atom Unpaired Electrons B) write the electron configuration. Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35. Find out the molecular geometry, hybridisation,. Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone pairs on each atom. Learn how to draw the lewis. Bromine Atom Unpaired Electrons.
From www.chegg.com
Solved For Bromine atom a) Determine the total number of Bromine Atom Unpaired Electrons Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. C) identify the core electrons and the. Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone pairs on each atom. One lone pair and three unpaired electrons. Learn how to draw the lewis structure of bromine, a. Bromine Atom Unpaired Electrons.
From stock.adobe.com
Br Bromine Element Information Facts, Properties, Trends, Uses and Bromine Atom Unpaired Electrons Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. Find out the molecular geometry, hybridisation,. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. A) determine the total number of unpaired electrons. Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and. Bromine Atom Unpaired Electrons.
From www.shutterstock.com
Symbol Electron Diagram Bromine Illustration Stock Vector (Royalty Free Bromine Atom Unpaired Electrons Based on the structures shown above, bromine contains 1 unpaired electron, and phosphorus has 3 unpaired electrons. Group 15 elements such as nitrogen have five valence electrons in the atomic lewis symbol: Find out the molecular geometry, hybridisation,. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. B) write the electron configuration. C). Bromine Atom Unpaired Electrons.
From www.numerade.com
SOLVED 24. Use spdf notation to indicate the electronic configuration Bromine Atom Unpaired Electrons Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone pairs on each atom. Learn about the number and arrangement of protons, neutrons and electrons in bromine, a halogen element with atomic number 35. Learn the difference between paramagnetism and diamagnetism, two types of magnetic forms of atoms and ions. One. Bromine Atom Unpaired Electrons.
From www.youtube.com
Electron Configuration of Bromine Br Lesson YouTube Bromine Atom Unpaired Electrons One lone pair and three unpaired electrons. Learn how to draw the lewis structure of bromine (br2), a diatomic molecule with one bond and three lone pairs on each atom. Find out its natural and unstable isotopes, electron configuration, oxidation states and applications. Learn how to draw the lewis structure of bromine, a diatomic molecule with 14 valence electrons. Based. Bromine Atom Unpaired Electrons.