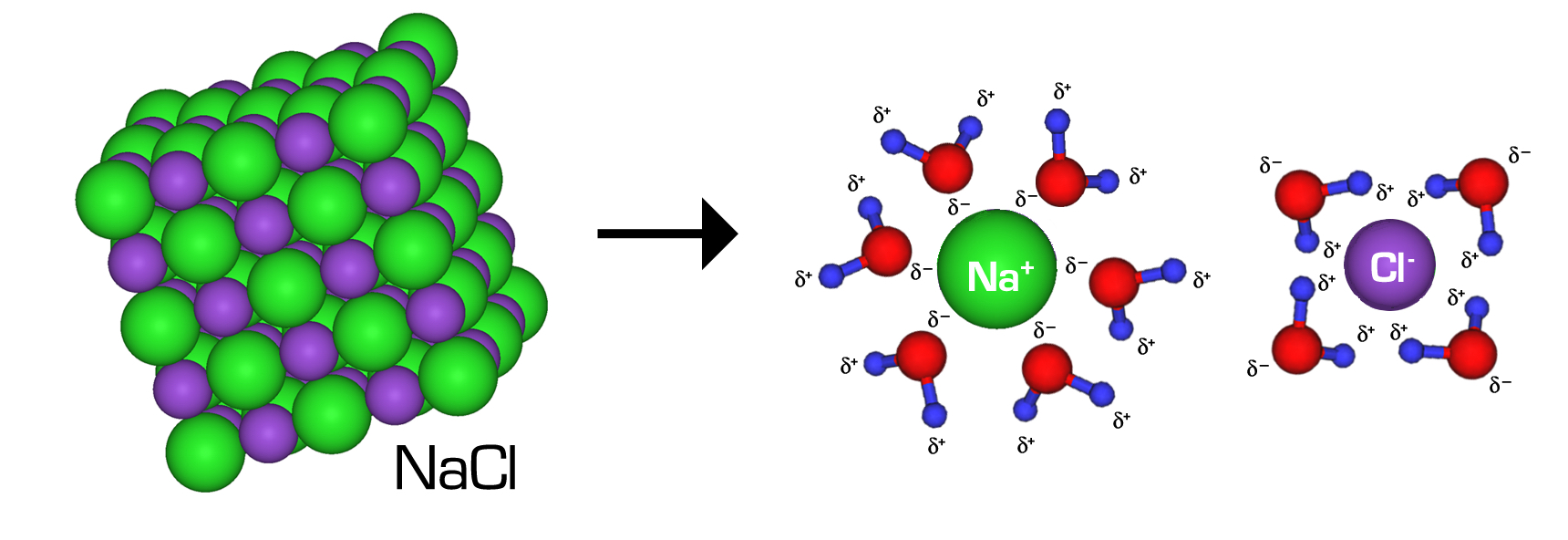
What Does Water Do To Ionic Compounds . Ionic compounds conduct an electric current when melted or dissolved in water. The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it is the ability of water to. When an ionic compound is dissolved in water a special interaction occurs. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The ionic bonds are broken and the. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water.
from www.visionlearning.com
The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. Ionic compounds conduct an electric current when melted or dissolved in water. The ionic bonds are broken and the. When an ionic compound is dissolved in water a special interaction occurs. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it is the ability of water to.
Solutions, Solubility, and Colligative Properties Chemistry
What Does Water Do To Ionic Compounds The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it is the ability of water to. When an ionic compound is dissolved in water a special interaction occurs. The ionic bonds are broken and the. Ionic compounds conduct an electric current when melted or dissolved in water. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation.
From www.slideserve.com
PPT Ionic Compounds and Metals PowerPoint Presentation, free download What Does Water Do To Ionic Compounds When an ionic compound is dissolved in water a special interaction occurs. Ionic compounds conduct an electric current when melted or dissolved in water. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The positive part of an ionic compound is attracted to the oxygen side of water. What Does Water Do To Ionic Compounds.
From sciencenotes.org
Net Ionic Equation and Complete Ionic Equation What Does Water Do To Ionic Compounds Ionic compounds conduct an electric current when melted or dissolved in water. The ionic bonds are broken and the. When an ionic compound is dissolved in water a special interaction occurs. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of. What Does Water Do To Ionic Compounds.
From www.sliderbase.com
Properties of Water Presentation Biology What Does Water Do To Ionic Compounds The ionic bonds are broken and the. Ionic compounds conduct an electric current when melted or dissolved in water. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. When ionic compounds are added to water, individual ions interact. What Does Water Do To Ionic Compounds.
From www.slideserve.com
PPT The Chemical & Physical Basis of Life PowerPoint Presentation What Does Water Do To Ionic Compounds The ionic bonds are broken and the. Ionic compounds conduct an electric current when melted or dissolved in water. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it. What Does Water Do To Ionic Compounds.
From www.youtube.com
How To Draw The Lewis Structures of Ionic Compounds YouTube What Does Water Do To Ionic Compounds When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The ionic bonds are broken and the. The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it is the ability of water to. Ionic compounds conduct an electric current. What Does Water Do To Ionic Compounds.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts What Does Water Do To Ionic Compounds The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it is the ability of water to. The ionic bonds are broken and the. When an ionic compound is dissolved in water a special interaction occurs. Ionic compounds conduct an electric current when melted or dissolved in water. The positive part. What Does Water Do To Ionic Compounds.
From www.slideserve.com
PPT Chapter 7 Reactions in Aqueous Solutions PowerPoint Presentation What Does Water Do To Ionic Compounds When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The ionic bonds are broken and the. Ionic compounds conduct an electric current when melted or dissolved in water. When an ionic compound is dissolved in water a special interaction occurs. The positive part of an ionic compound is. What Does Water Do To Ionic Compounds.
From www.slideserve.com
PPT The Nature of Aqueous Solutions and Molarity and Solution What Does Water Do To Ionic Compounds When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. The dynamic collection of water molecules surrounding an. What Does Water Do To Ionic Compounds.
From www.slideserve.com
PPT Solutions and Mixtures PowerPoint Presentation, free download What Does Water Do To Ionic Compounds When an ionic compound is dissolved in water a special interaction occurs. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The ionic bonds are broken and the. The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it. What Does Water Do To Ionic Compounds.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination What Does Water Do To Ionic Compounds The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. When an ionic compound is dissolved in water a special interaction occurs. Ionic compounds conduct an electric current when melted or dissolved in water. The dynamic collection of water. What Does Water Do To Ionic Compounds.
From general.chemistrysteps.com
Dissociation of Ionic Compounds Chemistry Steps What Does Water Do To Ionic Compounds When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. The ionic bonds are broken and the. The. What Does Water Do To Ionic Compounds.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID What Does Water Do To Ionic Compounds When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. Ionic compounds conduct an electric current when melted or dissolved in water. The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it is the ability of water to. The. What Does Water Do To Ionic Compounds.
From quizizz.com
Properties of ionic compounds Chemistry Quizizz What Does Water Do To Ionic Compounds The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. When an ionic compound is dissolved in water a special interaction occurs. Ionic compounds conduct an electric current when melted or dissolved in water. The ionic bonds are broken. What Does Water Do To Ionic Compounds.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID2276550 What Does Water Do To Ionic Compounds The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. When an ionic compound is dissolved in water. What Does Water Do To Ionic Compounds.
From sciencenotes.org
Ionic Bond Definition and Examples What Does Water Do To Ionic Compounds The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it is the ability of water to. The ionic bonds are broken and the. Ionic compounds conduct an electric current when melted or dissolved in water. When an ionic compound is dissolved in water a special interaction occurs. When ionic compounds. What Does Water Do To Ionic Compounds.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? What Does Water Do To Ionic Compounds When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. When an ionic compound is dissolved in water. What Does Water Do To Ionic Compounds.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision What Does Water Do To Ionic Compounds The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it is the ability of water to. When an ionic compound is dissolved in water a special interaction occurs. Ionic compounds conduct an electric current when melted or dissolved in water. The positive part of an ionic compound is attracted to. What Does Water Do To Ionic Compounds.
From www.slideserve.com
PPT The Ionic Product of Water PowerPoint Presentation, free download What Does Water Do To Ionic Compounds The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. Ionic compounds conduct an electric current when melted or dissolved in water. When an ionic compound is dissolved in water a special interaction occurs. The ionic bonds are broken. What Does Water Do To Ionic Compounds.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions What Does Water Do To Ionic Compounds When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it is the ability of water to. When an ionic compound is dissolved in water a special interaction occurs. The. What Does Water Do To Ionic Compounds.
From www.youtube.com
Predicting solubility of ionic compounds in water YouTube What Does Water Do To Ionic Compounds The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. When an ionic compound is dissolved in water a special interaction occurs. When ionic compounds are added to water, individual ions interact with the polar regions of the water. What Does Water Do To Ionic Compounds.
From www.slideserve.com
PPT Solubility of Ionic Compounds PowerPoint Presentation, free What Does Water Do To Ionic Compounds Ionic compounds conduct an electric current when melted or dissolved in water. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. When an ionic compound is dissolved in water a special interaction occurs. The ionic bonds are broken. What Does Water Do To Ionic Compounds.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download What Does Water Do To Ionic Compounds The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. When an ionic compound is dissolved in water a special interaction occurs. Ionic compounds conduct an electric current when melted or dissolved in water. The dynamic collection of water. What Does Water Do To Ionic Compounds.
From www.mrbarnestc.com
Ionic Equations Mr Barnes Teaches Chemistry What Does Water Do To Ionic Compounds When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. When an ionic compound is dissolved in water. What Does Water Do To Ionic Compounds.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 What Does Water Do To Ionic Compounds The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. Ionic compounds conduct an electric current when melted or dissolved in water. When ionic compounds are added to water, individual ions interact with the polar regions of the water. What Does Water Do To Ionic Compounds.
From www.teachoo.com
The table shown below gives information about four Chemistry MCQ What Does Water Do To Ionic Compounds The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it is the ability of water to. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. Ionic compounds conduct an electric current when melted or dissolved in water. The. What Does Water Do To Ionic Compounds.
From www.visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry What Does Water Do To Ionic Compounds The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it is the ability of water to. The ionic bonds. What Does Water Do To Ionic Compounds.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica What Does Water Do To Ionic Compounds When an ionic compound is dissolved in water a special interaction occurs. The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it is the ability of water to. Ionic compounds conduct an electric current when melted or dissolved in water. The ionic bonds are broken and the. When ionic compounds. What Does Water Do To Ionic Compounds.
From socratic.org
What is the chemical equation for HCl dissolving into water and What Does Water Do To Ionic Compounds The ionic bonds are broken and the. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. When an ionic compound is dissolved in water a special interaction occurs. Ionic compounds conduct an electric current when melted or dissolved. What Does Water Do To Ionic Compounds.
From en.wikipedia.org
Ionic bonding Wikipedia What Does Water Do To Ionic Compounds The ionic bonds are broken and the. The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it is the ability of water to. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen. What Does Water Do To Ionic Compounds.
From www.slideserve.com
PPT LECTURE 7 IONIC COMPOUNDS (Ch. 6) PowerPoint Presentation, free What Does Water Do To Ionic Compounds The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The dynamic collection of water molecules surrounding an. What Does Water Do To Ionic Compounds.
From www.britannica.com
Ionic bond Definition, Properties, Examples, & Facts Britannica What Does Water Do To Ionic Compounds When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. Ionic compounds conduct an electric current when melted or dissolved in water. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen. What Does Water Do To Ionic Compounds.
From www.youtube.com
Why are ionic compounds soluble in water? YouTube What Does Water Do To Ionic Compounds The ionic bonds are broken and the. The dynamic collection of water molecules surrounding an ion in solution is referred to as the solvation shell and it is the ability of water to. The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen. What Does Water Do To Ionic Compounds.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID2948816 What Does Water Do To Ionic Compounds The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. When ionic compounds are added to water, individual ions interact with the polar regions of the water molecules during the dissociation. The dynamic collection of water molecules surrounding an. What Does Water Do To Ionic Compounds.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 What Does Water Do To Ionic Compounds The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. Ionic compounds conduct an electric current when melted or dissolved in water. The ionic bonds are broken and the. When ionic compounds are added to water, individual ions interact. What Does Water Do To Ionic Compounds.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download What Does Water Do To Ionic Compounds The positive part of an ionic compound is attracted to the oxygen side of water while the negative portion of the compound is attracted to the hydrogen side of the water. The ionic bonds are broken and the. When an ionic compound is dissolved in water a special interaction occurs. The dynamic collection of water molecules surrounding an ion in. What Does Water Do To Ionic Compounds.