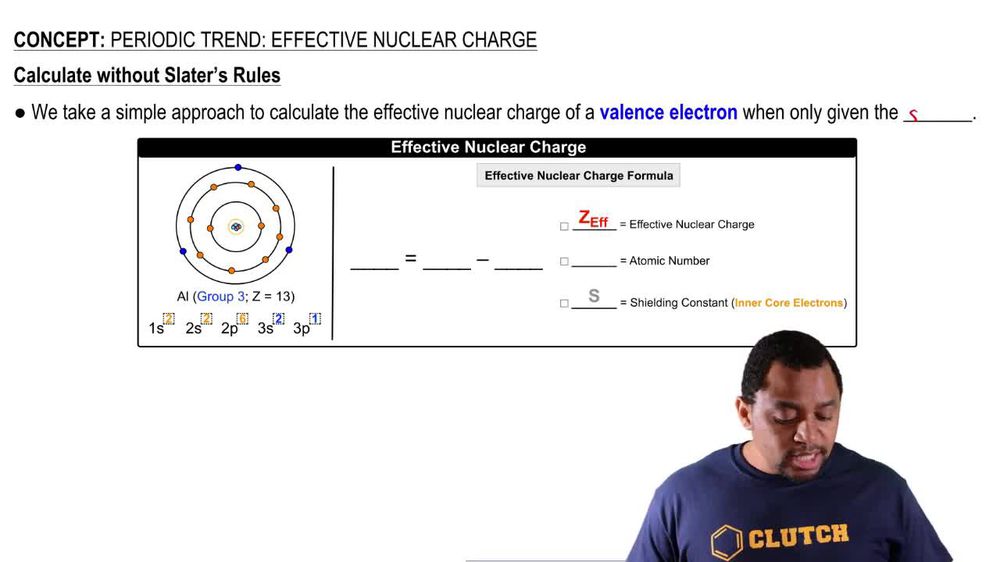
What Happens To Electron Shielding Down A Group . Electrons in an \ (s\) orbital can shield \ (p\). The effective nuclear charge can increase or decrease down a group. This is due to the shielding or screen effect of the outer electrons from the nucleus. In a neutral atom, the number. The stronger the attraction, the more. But, the atomic radius of the atoms increases as you are adding more shells of electrons, making the atoms bigger The number of protons in the atom is increased, so the nuclear charge increases; A measure of the attraction between the incoming electron and the nucleus. As the nuclear charge is well known, the effective charge. The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. Ionisation energy down a group. Going down a group, the ionisation energy decreases. The ionisation energy down a group decreases due to the following factors:
from www.pearson.com
In a neutral atom, the number. The ionisation energy down a group decreases due to the following factors: But, the atomic radius of the atoms increases as you are adding more shells of electrons, making the atoms bigger As the nuclear charge is well known, the effective charge. Ionisation energy down a group. The number of protons in the atom is increased, so the nuclear charge increases; This is due to the shielding or screen effect of the outer electrons from the nucleus. Electrons in an \ (s\) orbital can shield \ (p\). The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. A measure of the attraction between the incoming electron and the nucleus.
How does electron shielding in multielectron atoms give rise to e
What Happens To Electron Shielding Down A Group This is due to the shielding or screen effect of the outer electrons from the nucleus. A measure of the attraction between the incoming electron and the nucleus. The effective nuclear charge can increase or decrease down a group. Going down a group, the ionisation energy decreases. As the nuclear charge is well known, the effective charge. In a neutral atom, the number. The stronger the attraction, the more. The ionisation energy down a group decreases due to the following factors: The number of protons in the atom is increased, so the nuclear charge increases; But, the atomic radius of the atoms increases as you are adding more shells of electrons, making the atoms bigger The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. Electrons in an \ (s\) orbital can shield \ (p\). This is due to the shielding or screen effect of the outer electrons from the nucleus. Ionisation energy down a group.
From slideplayer.com
Coulomb’s Law PERIODIC TRENDS Nuclear Charge Electron Shielding ppt What Happens To Electron Shielding Down A Group Electrons in an \ (s\) orbital can shield \ (p\). A measure of the attraction between the incoming electron and the nucleus. The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. The number of protons in the atom is increased, so the nuclear. What Happens To Electron Shielding Down A Group.
From www.youtube.com
Shielding Effect in the Periodic Table Chemistry YouTube What Happens To Electron Shielding Down A Group Going down a group, the ionisation energy decreases. In a neutral atom, the number. The ionisation energy down a group decreases due to the following factors: Electrons in an \ (s\) orbital can shield \ (p\). The stronger the attraction, the more. The number of protons in the atom is increased, so the nuclear charge increases; But, the atomic radius. What Happens To Electron Shielding Down A Group.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision What Happens To Electron Shielding Down A Group The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. Electrons in an \ (s\) orbital can shield \ (p\). The ionisation energy down a group decreases due to the following factors: As the nuclear charge is well known, the effective charge. The stronger. What Happens To Electron Shielding Down A Group.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint What Happens To Electron Shielding Down A Group As the nuclear charge is well known, the effective charge. But, the atomic radius of the atoms increases as you are adding more shells of electrons, making the atoms bigger Going down a group, the ionisation energy decreases. This is due to the shielding or screen effect of the outer electrons from the nucleus. The stronger the attraction, the more.. What Happens To Electron Shielding Down A Group.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The What Happens To Electron Shielding Down A Group The effective nuclear charge can increase or decrease down a group. The number of protons in the atom is increased, so the nuclear charge increases; Electrons in an \ (s\) orbital can shield \ (p\). Going down a group, the ionisation energy decreases. This is due to the shielding or screen effect of the outer electrons from the nucleus. But,. What Happens To Electron Shielding Down A Group.
From slideplayer.com
Periodic Trends OBJECTIVES ppt download What Happens To Electron Shielding Down A Group As the nuclear charge is well known, the effective charge. Electrons in an \ (s\) orbital can shield \ (p\). A measure of the attraction between the incoming electron and the nucleus. This is due to the shielding or screen effect of the outer electrons from the nucleus. Going down a group, the ionisation energy decreases. The simple explaination (constant. What Happens To Electron Shielding Down A Group.
From www.slideshare.net
Periodic trends What Happens To Electron Shielding Down A Group Electrons in an \ (s\) orbital can shield \ (p\). The number of protons in the atom is increased, so the nuclear charge increases; The effective nuclear charge can increase or decrease down a group. But, the atomic radius of the atoms increases as you are adding more shells of electrons, making the atoms bigger This is due to the. What Happens To Electron Shielding Down A Group.
From anthonystrendsassignment.weebly.com
Electronegativity Periodic Trends What Happens To Electron Shielding Down A Group Ionisation energy down a group. Electrons in an \ (s\) orbital can shield \ (p\). A measure of the attraction between the incoming electron and the nucleus. The number of protons in the atom is increased, so the nuclear charge increases; The effective nuclear charge can increase or decrease down a group. As the nuclear charge is well known, the. What Happens To Electron Shielding Down A Group.
From ar.inspiredpencil.com
Shielding Effect Electrons What Happens To Electron Shielding Down A Group Electrons in an \ (s\) orbital can shield \ (p\). In a neutral atom, the number. A measure of the attraction between the incoming electron and the nucleus. But, the atomic radius of the atoms increases as you are adding more shells of electrons, making the atoms bigger This is due to the shielding or screen effect of the outer. What Happens To Electron Shielding Down A Group.
From www.youtube.com
Electron shielding YouTube What Happens To Electron Shielding Down A Group In a neutral atom, the number. This is due to the shielding or screen effect of the outer electrons from the nucleus. But, the atomic radius of the atoms increases as you are adding more shells of electrons, making the atoms bigger Going down a group, the ionisation energy decreases. The number of protons in the atom is increased, so. What Happens To Electron Shielding Down A Group.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 What Happens To Electron Shielding Down A Group In a neutral atom, the number. A measure of the attraction between the incoming electron and the nucleus. This is due to the shielding or screen effect of the outer electrons from the nucleus. Electrons in an \ (s\) orbital can shield \ (p\). The number of protons in the atom is increased, so the nuclear charge increases; The stronger. What Happens To Electron Shielding Down A Group.
From www.slideserve.com
PPT Chapter 6 Chemical Bonding PowerPoint Presentation, free What Happens To Electron Shielding Down A Group Ionisation energy down a group. Electrons in an \ (s\) orbital can shield \ (p\). The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. The ionisation energy down a group decreases due to the following factors: This is due to the shielding or. What Happens To Electron Shielding Down A Group.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e What Happens To Electron Shielding Down A Group As the nuclear charge is well known, the effective charge. A measure of the attraction between the incoming electron and the nucleus. Ionisation energy down a group. Electrons in an \ (s\) orbital can shield \ (p\). The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons. What Happens To Electron Shielding Down A Group.
From general.chemistrysteps.com
Ionization energy Chemistry Steps What Happens To Electron Shielding Down A Group As the nuclear charge is well known, the effective charge. In a neutral atom, the number. Electrons in an \ (s\) orbital can shield \ (p\). The number of protons in the atom is increased, so the nuclear charge increases; Going down a group, the ionisation energy decreases. The stronger the attraction, the more. But, the atomic radius of the. What Happens To Electron Shielding Down A Group.
From www.slideserve.com
PPT Chapter 6 Ionic Bonds and Some Main Group Chemistry PowerPoint What Happens To Electron Shielding Down A Group The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. In a neutral atom, the number. Ionisation energy down a group. As the nuclear charge is well known, the effective charge. The effective nuclear charge can increase or decrease down a group. This is. What Happens To Electron Shielding Down A Group.
From www.slideshare.net
Lesson 1 Group 7 Elements Eam What Happens To Electron Shielding Down A Group But, the atomic radius of the atoms increases as you are adding more shells of electrons, making the atoms bigger The number of protons in the atom is increased, so the nuclear charge increases; The stronger the attraction, the more. The ionisation energy down a group decreases due to the following factors: As the nuclear charge is well known, the. What Happens To Electron Shielding Down A Group.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID What Happens To Electron Shielding Down A Group Ionisation energy down a group. The effective nuclear charge can increase or decrease down a group. A measure of the attraction between the incoming electron and the nucleus. The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. This is due to the shielding. What Happens To Electron Shielding Down A Group.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding What Happens To Electron Shielding Down A Group As the nuclear charge is well known, the effective charge. The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. A measure of the attraction between the incoming electron and the nucleus. In a neutral atom, the number. The effective nuclear charge can increase. What Happens To Electron Shielding Down A Group.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID3652853 What Happens To Electron Shielding Down A Group The number of protons in the atom is increased, so the nuclear charge increases; The stronger the attraction, the more. A measure of the attraction between the incoming electron and the nucleus. Electrons in an \ (s\) orbital can shield \ (p\). As the nuclear charge is well known, the effective charge. In a neutral atom, the number. The ionisation. What Happens To Electron Shielding Down A Group.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The What Happens To Electron Shielding Down A Group The effective nuclear charge can increase or decrease down a group. The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. The number of protons in the atom is increased, so the nuclear charge increases; In a neutral atom, the number. This is due. What Happens To Electron Shielding Down A Group.
From www.slideserve.com
PPT Chapter 6 The Periodic Table PowerPoint Presentation, free What Happens To Electron Shielding Down A Group The number of protons in the atom is increased, so the nuclear charge increases; In a neutral atom, the number. Electrons in an \ (s\) orbital can shield \ (p\). As the nuclear charge is well known, the effective charge. Ionisation energy down a group. The effective nuclear charge can increase or decrease down a group. But, the atomic radius. What Happens To Electron Shielding Down A Group.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e What Happens To Electron Shielding Down A Group Ionisation energy down a group. The number of protons in the atom is increased, so the nuclear charge increases; The effective nuclear charge can increase or decrease down a group. As the nuclear charge is well known, the effective charge. The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the. What Happens To Electron Shielding Down A Group.
From slideplayer.com
Coulomb’s Law PERIODIC TRENDS Nuclear Charge Electron Shielding ppt What Happens To Electron Shielding Down A Group The number of protons in the atom is increased, so the nuclear charge increases; As the nuclear charge is well known, the effective charge. The stronger the attraction, the more. The effective nuclear charge can increase or decrease down a group. Going down a group, the ionisation energy decreases. But, the atomic radius of the atoms increases as you are. What Happens To Electron Shielding Down A Group.
From www.toppr.com
How are shielding effect and atomic radius related? What Happens To Electron Shielding Down A Group Going down a group, the ionisation energy decreases. The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. In a neutral atom, the number. A measure of the attraction between the incoming electron and the nucleus. The stronger the attraction, the more. As the. What Happens To Electron Shielding Down A Group.
From byjus.com
What is shielding and deshielding in NMR? Give an example? What Happens To Electron Shielding Down A Group A measure of the attraction between the incoming electron and the nucleus. The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. The ionisation energy down a group decreases due to the following factors: Going down a group, the ionisation energy decreases. The stronger. What Happens To Electron Shielding Down A Group.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID5583827 What Happens To Electron Shielding Down A Group In a neutral atom, the number. This is due to the shielding or screen effect of the outer electrons from the nucleus. But, the atomic radius of the atoms increases as you are adding more shells of electrons, making the atoms bigger The effective nuclear charge can increase or decrease down a group. A measure of the attraction between the. What Happens To Electron Shielding Down A Group.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube What Happens To Electron Shielding Down A Group In a neutral atom, the number. The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. The number of protons in the atom is increased, so the nuclear charge increases; The effective nuclear charge can increase or decrease down a group. The stronger the. What Happens To Electron Shielding Down A Group.
From studymind.co.uk
Group 1 Reactivity (GCSE Chemistry) Study Mind What Happens To Electron Shielding Down A Group This is due to the shielding or screen effect of the outer electrons from the nucleus. Ionisation energy down a group. The ionisation energy down a group decreases due to the following factors: Electrons in an \ (s\) orbital can shield \ (p\). The effective nuclear charge can increase or decrease down a group. In a neutral atom, the number.. What Happens To Electron Shielding Down A Group.
From www.slideserve.com
PPT Chapter 6 Periodic Table PowerPoint Presentation, free download What Happens To Electron Shielding Down A Group A measure of the attraction between the incoming electron and the nucleus. In a neutral atom, the number. But, the atomic radius of the atoms increases as you are adding more shells of electrons, making the atoms bigger Ionisation energy down a group. Electrons in an \ (s\) orbital can shield \ (p\). The effective nuclear charge can increase or. What Happens To Electron Shielding Down A Group.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download What Happens To Electron Shielding Down A Group Going down a group, the ionisation energy decreases. In a neutral atom, the number. The stronger the attraction, the more. Ionisation energy down a group. The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. The ionisation energy down a group decreases due to. What Happens To Electron Shielding Down A Group.
From slideplayer.com
The Periodic Table Chapter ppt download What Happens To Electron Shielding Down A Group The ionisation energy down a group decreases due to the following factors: A measure of the attraction between the incoming electron and the nucleus. The number of protons in the atom is increased, so the nuclear charge increases; This is due to the shielding or screen effect of the outer electrons from the nucleus. Going down a group, the ionisation. What Happens To Electron Shielding Down A Group.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity What Happens To Electron Shielding Down A Group As the nuclear charge is well known, the effective charge. The effective nuclear charge can increase or decrease down a group. In a neutral atom, the number. The stronger the attraction, the more. Electrons in an \ (s\) orbital can shield \ (p\). Ionisation energy down a group. The simple explaination (constant zeff z e f f down the group). What Happens To Electron Shielding Down A Group.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision What Happens To Electron Shielding Down A Group Electrons in an \ (s\) orbital can shield \ (p\). In a neutral atom, the number. The stronger the attraction, the more. But, the atomic radius of the atoms increases as you are adding more shells of electrons, making the atoms bigger The number of protons in the atom is increased, so the nuclear charge increases; Ionisation energy down a. What Happens To Electron Shielding Down A Group.
From mammothmemory.net
As you move down group 1 and 7 elements get more reactive What Happens To Electron Shielding Down A Group The stronger the attraction, the more. As the nuclear charge is well known, the effective charge. But, the atomic radius of the atoms increases as you are adding more shells of electrons, making the atoms bigger The ionisation energy down a group decreases due to the following factors: A measure of the attraction between the incoming electron and the nucleus.. What Happens To Electron Shielding Down A Group.
From chemistnotes.com
Shielding Effect or Screening Effect Definition, Factors Affecting What Happens To Electron Shielding Down A Group Going down a group, the ionisation energy decreases. The ionisation energy down a group decreases due to the following factors: Ionisation energy down a group. The simple explaination (constant zeff z e f f down the group) assumes that all inner electrons exactly cancel the effect of protons in the nucleus. The effective nuclear charge can increase or decrease down. What Happens To Electron Shielding Down A Group.