Atomic Radius Of Chlorine And Argon . These radii are generally not the same (figure 8.2.2d 8.2. description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). Below mentioned radii are the van der. this webelements periodic table page contains radii of atoms and ions for the element argon. Because argon is to the right of chlorine. compare chlorine and argon on the basis of their properties, attributes and periodic table facts. according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. Compare elements on more than 90 properties. 119 rows atomic radius of all the elements are mentioned in the chart below. an atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for example, cl2(s) cl 2 (s) at low temperatures). this will mean that adding a proton and electron (or going right in a period) will decrease the atomic radius.
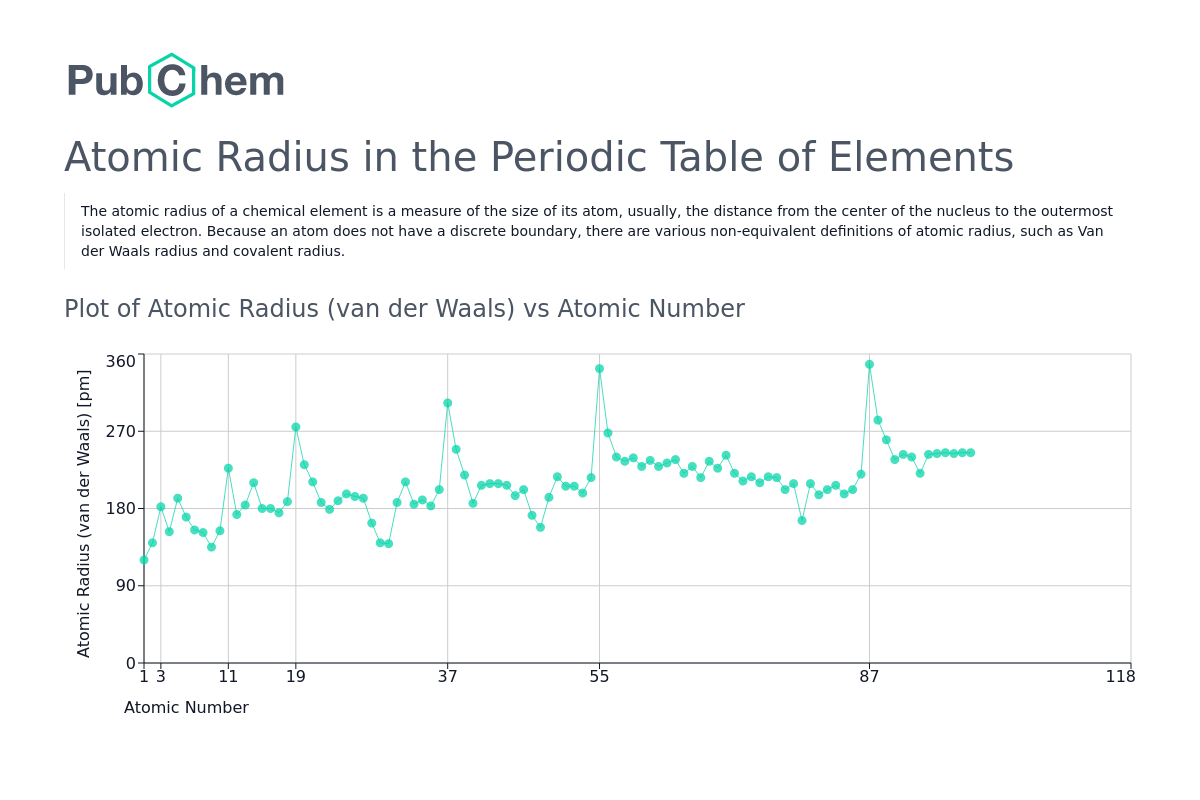
from pubchem.ncbi.nlm.nih.gov
compare chlorine and argon on the basis of their properties, attributes and periodic table facts. Because argon is to the right of chlorine. according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. 119 rows atomic radius of all the elements are mentioned in the chart below. Compare elements on more than 90 properties. an atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for example, cl2(s) cl 2 (s) at low temperatures). These radii are generally not the same (figure 8.2.2d 8.2. this webelements periodic table page contains radii of atoms and ions for the element argon. Below mentioned radii are the van der. description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon).
Atomic Radius Periodic Table of Elements PubChem
Atomic Radius Of Chlorine And Argon according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. 119 rows atomic radius of all the elements are mentioned in the chart below. These radii are generally not the same (figure 8.2.2d 8.2. Compare elements on more than 90 properties. according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. an atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for example, cl2(s) cl 2 (s) at low temperatures). Because argon is to the right of chlorine. this will mean that adding a proton and electron (or going right in a period) will decrease the atomic radius. description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). this webelements periodic table page contains radii of atoms and ions for the element argon. Below mentioned radii are the van der. compare chlorine and argon on the basis of their properties, attributes and periodic table facts.
From socratic.org
How does the atomic radius of argon compare to that of chlorine? Socratic Atomic Radius Of Chlorine And Argon description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. an atom such as chlorine has both a covalent radius (the distance between the two atoms in. Atomic Radius Of Chlorine And Argon.
From www.animalia-life.club
Atomic Radius Diagram Atomic Radius Of Chlorine And Argon Because argon is to the right of chlorine. Below mentioned radii are the van der. description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. this webelements. Atomic Radius Of Chlorine And Argon.
From schematicdatajudder88.z22.web.core.windows.net
Chlorine Atomic Structure Diagram Atomic Radius Of Chlorine And Argon according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). 119 rows atomic radius of all the elements are mentioned in the chart below. this will. Atomic Radius Of Chlorine And Argon.
From maddisonbarrett.z21.web.core.windows.net
Chart Of Atomic Radius Atomic Radius Of Chlorine And Argon Below mentioned radii are the van der. this will mean that adding a proton and electron (or going right in a period) will decrease the atomic radius. this webelements periodic table page contains radii of atoms and ions for the element argon. description and explanation of the trend in atomic radius going across period 3 in the. Atomic Radius Of Chlorine And Argon.
From sklep.foteks.pl
Plakat types of atomic radius of a chemical element. Nitrogen, oxygen Atomic Radius Of Chlorine And Argon 119 rows atomic radius of all the elements are mentioned in the chart below. this will mean that adding a proton and electron (or going right in a period) will decrease the atomic radius. Compare elements on more than 90 properties. Because argon is to the right of chlorine. an atom such as chlorine has both a. Atomic Radius Of Chlorine And Argon.
From learningschoolsurveis9.z4.web.core.windows.net
Atomic Radius Trend Examples Atomic Radius Of Chlorine And Argon Because argon is to the right of chlorine. Below mentioned radii are the van der. this will mean that adding a proton and electron (or going right in a period) will decrease the atomic radius. according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. this webelements. Atomic Radius Of Chlorine And Argon.
From saylordotorg.github.io
The Periodic Table and Periodic Trends Atomic Radius Of Chlorine And Argon this will mean that adding a proton and electron (or going right in a period) will decrease the atomic radius. description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). Below mentioned radii are the van der. this webelements periodic table page contains radii of atoms and. Atomic Radius Of Chlorine And Argon.
From pubchem.ncbi.nlm.nih.gov
Atomic Radius Periodic Table of Elements PubChem Atomic Radius Of Chlorine And Argon Because argon is to the right of chlorine. according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. this webelements periodic table page contains radii of atoms and ions for the element argon. compare chlorine and argon on the basis of their properties, attributes and periodic table. Atomic Radius Of Chlorine And Argon.
From quizgrouchiest.z4.web.core.windows.net
How To Calculate Atomic Radius Atomic Radius Of Chlorine And Argon 119 rows atomic radius of all the elements are mentioned in the chart below. this webelements periodic table page contains radii of atoms and ions for the element argon. description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). These radii are generally not the same (figure. Atomic Radius Of Chlorine And Argon.
From favpng.com
Atom Bohr Model Electron Configuration Chlorine, PNG, 1000x1000px, Atom Atomic Radius Of Chlorine And Argon description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). Compare elements on more than 90 properties. These radii are generally not the same (figure 8.2.2d 8.2. an atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2. Atomic Radius Of Chlorine And Argon.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Atomic Radius Of Chlorine And Argon description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). 119 rows atomic radius of all the elements are mentioned in the chart below. this webelements periodic table page contains radii of atoms and ions for the element argon. compare chlorine and argon on the basis. Atomic Radius Of Chlorine And Argon.
From periodictableguide.com
Get the Periodic table with Atomic radius values (Img+Chart) Atomic Radius Of Chlorine And Argon according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. 119 rows atomic radius of all the elements are mentioned in the chart below. this will mean that adding a proton and electron (or going right in a period) will decrease the atomic radius. this webelements. Atomic Radius Of Chlorine And Argon.
From www.shutterstock.com
Types Atomic Radius Chemical Element Atomic Stock Vector (Royalty Free Atomic Radius Of Chlorine And Argon this will mean that adding a proton and electron (or going right in a period) will decrease the atomic radius. These radii are generally not the same (figure 8.2.2d 8.2. Below mentioned radii are the van der. compare chlorine and argon on the basis of their properties, attributes and periodic table facts. Compare elements on more than 90. Atomic Radius Of Chlorine And Argon.
From brokeasshome.com
Periodic Table Of Elements Sorted By Atomic Radius Atomic Radius Of Chlorine And Argon according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. Compare elements on more than 90 properties. These radii are generally not the same (figure 8.2.2d 8.2. this will mean that adding a proton and electron (or going right in a period) will decrease the atomic radius. . Atomic Radius Of Chlorine And Argon.
From www.dreamstime.com
Atomic Radius Measurements of Diatomic Molecules Stock Vector Atomic Radius Of Chlorine And Argon Below mentioned radii are the van der. Compare elements on more than 90 properties. These radii are generally not the same (figure 8.2.2d 8.2. description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). an atom such as chlorine has both a covalent radius (the distance between the. Atomic Radius Of Chlorine And Argon.
From stock.adobe.com
Sulfur, Chlorine, Argon on the periodic table of the elements on black Atomic Radius Of Chlorine And Argon an atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms in different molecules in, for example, cl2(s) cl 2 (s) at low temperatures). compare chlorine and argon on the basis of their properties, attributes. Atomic Radius Of Chlorine And Argon.
From www.nuclear-power.com
Chlorine Atomic Number Atomic Mass Density of Chlorine nuclear Atomic Radius Of Chlorine And Argon description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). Because argon is to the right of chlorine. These radii are generally not the same (figure 8.2.2d 8.2. Compare elements on more than 90 properties. Below mentioned radii are the van der. according to the periodic table, chlorine. Atomic Radius Of Chlorine And Argon.
From pediabay.com
Atomic Radius of Elements (With Periodic table Chart) Pediabay Atomic Radius Of Chlorine And Argon Compare elements on more than 90 properties. 119 rows atomic radius of all the elements are mentioned in the chart below. this webelements periodic table page contains radii of atoms and ions for the element argon. compare chlorine and argon on the basis of their properties, attributes and periodic table facts. an atom such as chlorine. Atomic Radius Of Chlorine And Argon.
From general.chemistrysteps.com
Atomic Radius Chemistry Steps Atomic Radius Of Chlorine And Argon this will mean that adding a proton and electron (or going right in a period) will decrease the atomic radius. 119 rows atomic radius of all the elements are mentioned in the chart below. according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. an atom. Atomic Radius Of Chlorine And Argon.
From circuitenginecutty88.z22.web.core.windows.net
Orbital Diagram Of Chlorine Atomic Radius Of Chlorine And Argon description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). this webelements periodic table page contains radii of atoms and ions for the element argon. Compare elements on more than 90 properties. 119 rows atomic radius of all the elements are mentioned in the chart below. . Atomic Radius Of Chlorine And Argon.
From sciencenotes.org
Atomic Radius and Ionic Radius Atomic Radius Of Chlorine And Argon compare chlorine and argon on the basis of their properties, attributes and periodic table facts. Compare elements on more than 90 properties. this webelements periodic table page contains radii of atoms and ions for the element argon. Below mentioned radii are the van der. description and explanation of the trend in atomic radius going across period 3. Atomic Radius Of Chlorine And Argon.
From chem.libretexts.org
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic Atomic Radius Of Chlorine And Argon this will mean that adding a proton and electron (or going right in a period) will decrease the atomic radius. according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. 119 rows atomic radius of all the elements are mentioned in the chart below. compare chlorine. Atomic Radius Of Chlorine And Argon.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Atomic Radius Of Chlorine And Argon 119 rows atomic radius of all the elements are mentioned in the chart below. These radii are generally not the same (figure 8.2.2d 8.2. Below mentioned radii are the van der. Because argon is to the right of chlorine. according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18. Atomic Radius Of Chlorine And Argon.
From socratic.org
How does the atomic radius of argon compare to that of chlorine? Socratic Atomic Radius Of Chlorine And Argon These radii are generally not the same (figure 8.2.2d 8.2. this webelements periodic table page contains radii of atoms and ions for the element argon. according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. an atom such as chlorine has both a covalent radius (the distance. Atomic Radius Of Chlorine And Argon.
From socratic.org
How does the atomic radius of argon compare to that of chlorine? Socratic Atomic Radius Of Chlorine And Argon Because argon is to the right of chlorine. 119 rows atomic radius of all the elements are mentioned in the chart below. this webelements periodic table page contains radii of atoms and ions for the element argon. These radii are generally not the same (figure 8.2.2d 8.2. compare chlorine and argon on the basis of their properties,. Atomic Radius Of Chlorine And Argon.
From material-properties.org
Argon Periodic Table and Atomic Properties Atomic Radius Of Chlorine And Argon Below mentioned radii are the van der. compare chlorine and argon on the basis of their properties, attributes and periodic table facts. according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. These radii are generally not the same (figure 8.2.2d 8.2. description and explanation of the. Atomic Radius Of Chlorine And Argon.
From www.doubtnut.com
(a) Give the schematic atomic structures of chlorine atom and chloride Atomic Radius Of Chlorine And Argon Compare elements on more than 90 properties. These radii are generally not the same (figure 8.2.2d 8.2. Below mentioned radii are the van der. this webelements periodic table page contains radii of atoms and ions for the element argon. compare chlorine and argon on the basis of their properties, attributes and periodic table facts. this will mean. Atomic Radius Of Chlorine And Argon.
From www.webelements.com
Elements Periodic Table » Chlorine » properties of free atoms Atomic Radius Of Chlorine And Argon according to the periodic table, chlorine is located in group 17 17 whereas argon is located in group 18 18. Below mentioned radii are the van der. an atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between. Atomic Radius Of Chlorine And Argon.
From courses.lumenlearning.com
Electrons Biology for Majors I Atomic Radius Of Chlorine And Argon description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). These radii are generally not the same (figure 8.2.2d 8.2. this webelements periodic table page contains radii of atoms and ions for the element argon. this will mean that adding a proton and electron (or going right. Atomic Radius Of Chlorine And Argon.
From bradleyrahman.z13.web.core.windows.net
Chart Of Atomic Radius Of Elements Atomic Radius Of Chlorine And Argon Because argon is to the right of chlorine. Below mentioned radii are the van der. These radii are generally not the same (figure 8.2.2d 8.2. an atom such as chlorine has both a covalent radius (the distance between the two atoms in a cl2 cl 2 molecule) and a van der waals radius (the distance between two cl atoms. Atomic Radius Of Chlorine And Argon.
From sciencenotes.org
Argon Facts Atomic Radius Of Chlorine And Argon Below mentioned radii are the van der. These radii are generally not the same (figure 8.2.2d 8.2. description and explanation of the trend in atomic radius going across period 3 in the periodic table (sodium to argon). this webelements periodic table page contains radii of atoms and ions for the element argon. according to the periodic table,. Atomic Radius Of Chlorine And Argon.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Atomic Radius Of Chlorine And Argon These radii are generally not the same (figure 8.2.2d 8.2. compare chlorine and argon on the basis of their properties, attributes and periodic table facts. this will mean that adding a proton and electron (or going right in a period) will decrease the atomic radius. this webelements periodic table page contains radii of atoms and ions for. Atomic Radius Of Chlorine And Argon.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Atomic Radius Of Chlorine And Argon These radii are generally not the same (figure 8.2.2d 8.2. 119 rows atomic radius of all the elements are mentioned in the chart below. compare chlorine and argon on the basis of their properties, attributes and periodic table facts. this webelements periodic table page contains radii of atoms and ions for the element argon. Below mentioned radii. Atomic Radius Of Chlorine And Argon.
From www.numerade.com
SOLVED Using only the periodic table arrange the following elements in Atomic Radius Of Chlorine And Argon Compare elements on more than 90 properties. Because argon is to the right of chlorine. These radii are generally not the same (figure 8.2.2d 8.2. Below mentioned radii are the van der. this will mean that adding a proton and electron (or going right in a period) will decrease the atomic radius. this webelements periodic table page contains. Atomic Radius Of Chlorine And Argon.
From learningschoolringso4rzh.z22.web.core.windows.net
Atomic Radius Trend Explained Atomic Radius Of Chlorine And Argon this webelements periodic table page contains radii of atoms and ions for the element argon. this will mean that adding a proton and electron (or going right in a period) will decrease the atomic radius. compare chlorine and argon on the basis of their properties, attributes and periodic table facts. according to the periodic table, chlorine. Atomic Radius Of Chlorine And Argon.