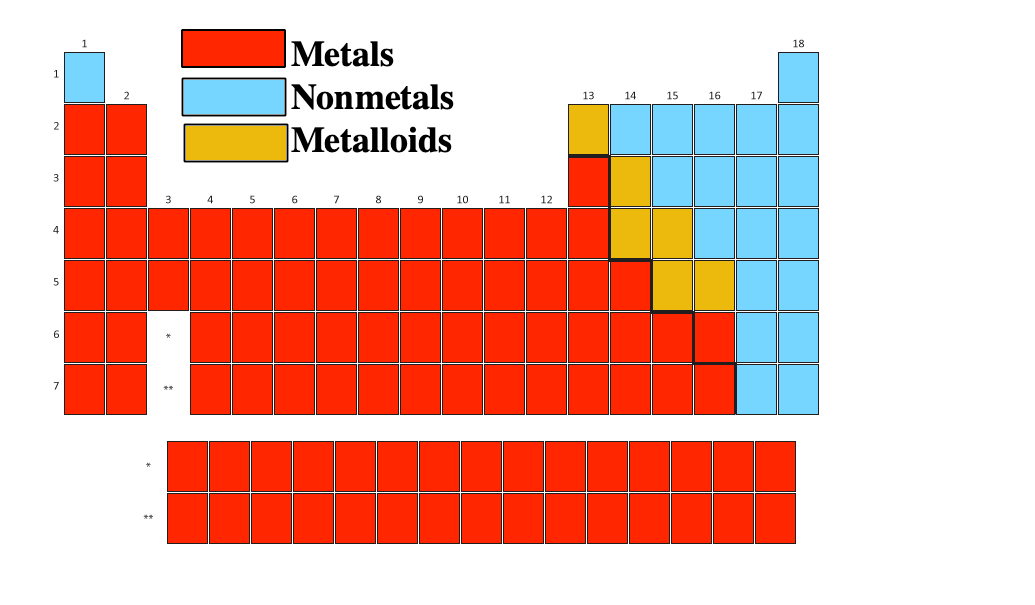
Metalloids And Amphoteric . most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Thus metals are electropositive elements. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. metalloids often act as semiconductors, making them important in electronics. metalloids are useful in the semiconductor industry. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. The physical appearance of metalloids can be. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. Metalloids have semiconductor properties and form amphoteric oxides. They can form alloys with. Metalloids are all solid at room temperature.
from www.animalia-life.club
The physical appearance of metalloids can be. metalloids are useful in the semiconductor industry. Metalloids are all solid at room temperature. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Thus metals are electropositive elements. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. They can form alloys with. metalloids often act as semiconductors, making them important in electronics. Metalloids have semiconductor properties and form amphoteric oxides. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of.
Periodic Table With Metals Nonmetals And Metalloids
Metalloids And Amphoteric most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. The physical appearance of metalloids can be. Thus metals are electropositive elements. They can form alloys with. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. Metalloids have semiconductor properties and form amphoteric oxides. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metalloids are all solid at room temperature. metalloids often act as semiconductors, making them important in electronics. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. metalloids are useful in the semiconductor industry.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation ID3842068 Metalloids And Amphoteric Metalloids are all solid at room temperature. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. metalloids often act as semiconductors, making them important in electronics. metalloids are useful in the semiconductor industry. They can form alloys with. Metalloids have semiconductor properties and form amphoteric oxides. They are. Metalloids And Amphoteric.
From www.slideserve.com
PPT Solubility of metal hydroxides, and amphoteric behavior Metalloids And Amphoteric Metalloids are all solid at room temperature. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. metalloids often act as semiconductors, making them important in electronics. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Metalloids have semiconductor properties. Metalloids And Amphoteric.
From www.youtube.com
Learning Tricks for liquid, metalloid, amphoteric elements and all Metalloids And Amphoteric They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. The physical appearance of metalloids can be. Thus metals are electropositive elements. metalloids often act as semiconductors, making them important in electronics. . Metalloids And Amphoteric.
From www.animalia-life.club
Periodic Table With Metals Nonmetals And Metalloids Metalloids And Amphoteric Thus metals are electropositive elements. Metalloids have semiconductor properties and form amphoteric oxides. metalloids are useful in the semiconductor industry. metalloids often act as semiconductors, making them important in electronics. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. the dividing line between metals and nonmetals can. Metalloids And Amphoteric.
From thechemistrynotes.com
Metalloids Definition, Properties, Uses, and Applications Metalloids And Amphoteric most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. metalloids are useful in the semiconductor industry. Metalloids are all solid at room temperature. They can form alloys with. Metalloids have semiconductor properties and form amphoteric oxides. metalloids often act as semiconductors, making them important in electronics. They are. Metalloids And Amphoteric.
From homedeso.vercel.app
Metals And Metalloids On Periodic Table Metalloids And Amphoteric They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. metalloids often act as semiconductors, making them important in electronics. Metalloids are all solid at room temperature. metalloids are useful in the semiconductor industry. Metalloids have semiconductor properties and form amphoteric oxides. They can form alloys with. the. Metalloids And Amphoteric.
From www.chemistrylearner.com
Metalloids Chemistry Learner Metalloids And Amphoteric Metalloids are all solid at room temperature. metalloids are useful in the semiconductor industry. Metalloids have semiconductor properties and form amphoteric oxides. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. They can form alloys with. the dividing line between metals and nonmetals can be found, in varying. Metalloids And Amphoteric.
From www.xometry.com
7 Elements of Metalloids Differences and Uses Xometry Metalloids And Amphoteric Metalloids are all solid at room temperature. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metalloids have semiconductor properties and form amphoteric oxides. The physical appearance of metalloids can be. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. They can form. Metalloids And Amphoteric.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Metalloids And Amphoteric metalloids often act as semiconductors, making them important in electronics. They can form alloys with. metalloids are useful in the semiconductor industry. Thus metals are electropositive elements. Metalloids are all solid at room temperature. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. The physical appearance of metalloids. Metalloids And Amphoteric.
From slideplayer.com
CHEM 120 WEEK 12 LECTURES WEEK 3) ppt download Metalloids And Amphoteric most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. metalloids often act as semiconductors, making them important in electronics. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. the dividing line between metals and nonmetals can be found,. Metalloids And Amphoteric.
From www.youtube.com
Amphoteric Molecules YouTube Metalloids And Amphoteric most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. The physical appearance of metalloids can be. Thus metals are electropositive elements. metalloids are useful in the semiconductor industry. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metalloids have semiconductor properties. Metalloids And Amphoteric.
From www.slideserve.com
PPT Unit 7 Acids and Bases PowerPoint Presentation, free download Metalloids And Amphoteric The physical appearance of metalloids can be. They can form alloys with. Thus metals are electropositive elements. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. metalloids often act as semiconductors, making them important in electronics. Metalloids are all solid at room temperature. They are characterized by bright luster, hardness,. Metalloids And Amphoteric.
From slideplayer.com
The Periodic Table Metals, Metalloids, and Nonmetals. ppt download Metalloids And Amphoteric Thus metals are electropositive elements. metalloids often act as semiconductors, making them important in electronics. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. They can form alloys with. metalloids are useful in the semiconductor industry. Metalloids have semiconductor properties and form amphoteric oxides. All elements except hydrogen, which. Metalloids And Amphoteric.
From fr.slideserve.com
PPT Acid and Bases PowerPoint Presentation, free download ID6947838 Metalloids And Amphoteric All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metalloids have semiconductor properties and form amphoteric oxides. Metalloids are all solid at room temperature. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. most metalloids have a shiny, metallic appearance but are. Metalloids And Amphoteric.
From www.youtube.com
Amphoteric oxides class 10 th ,metal and non metals YouTube Metalloids And Amphoteric Metalloids are all solid at room temperature. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Metalloids have semiconductor properties and form amphoteric oxides. metalloids are useful in the semiconductor industry. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of.. Metalloids And Amphoteric.
From www.youtube.com
12OR29 Amino Acids Amphoteric nature and zwitterions YouTube Metalloids And Amphoteric metalloids are useful in the semiconductor industry. The physical appearance of metalloids can be. Metalloids have semiconductor properties and form amphoteric oxides. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. Thus metals are electropositive elements. They are characterized by bright luster, hardness, ability to resonate sound and are. Metalloids And Amphoteric.
From www.youtube.com
What are Amphoteric oxides How are amphoteric oxides related to Metalloids And Amphoteric They can form alloys with. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. metalloids are useful in the semiconductor industry. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. They are characterized by bright luster, hardness, ability to resonate. Metalloids And Amphoteric.
From www.slideserve.com
PPT Solubility of metal hydroxides, and amphoteric behavior Metalloids And Amphoteric The physical appearance of metalloids can be. They can form alloys with. Thus metals are electropositive elements. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. the dividing line between metals and. Metalloids And Amphoteric.
From www.youtube.com
Amphoteric Oxides Metals and Non Metals Class 10 Science Chemistry Metalloids And Amphoteric They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. The physical appearance of metalloids can be. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display. Metalloids And Amphoteric.
From utedzz.blogspot.com
Periodic Table Metals Nonmetals And Metalloids 2019 Periodic Table Metalloids And Amphoteric Thus metals are electropositive elements. Metalloids are all solid at room temperature. The physical appearance of metalloids can be. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. metalloids often act as semiconductors,. Metalloids And Amphoteric.
From www.slideserve.com
PPT Chapter 15 Acids and Bases PowerPoint Presentation, free Metalloids And Amphoteric All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. Thus metals are electropositive elements. . Metalloids And Amphoteric.
From sciencenotes.org
Metalloids Science Notes and Projects Metalloids And Amphoteric Thus metals are electropositive elements. metalloids are useful in the semiconductor industry. They can form alloys with. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metalloids have semiconductor properties and form amphoteric oxides. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic. Metalloids And Amphoteric.
From www.thoughtco.com
Amphoteric Definition and Examples Metalloids And Amphoteric Thus metals are electropositive elements. metalloids often act as semiconductors, making them important in electronics. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. They can form alloys with. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. Metalloids have. Metalloids And Amphoteric.
From slideplayer.com
Periodic Relationships Among the Elements ppt download Metalloids And Amphoteric Metalloids are all solid at room temperature. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metalloids have semiconductor properties and form amphoteric oxides. The physical appearance of metalloids can be. metalloids are useful in the semiconductor industry. metalloids often act as semiconductors, making them important in electronics. most. Metalloids And Amphoteric.
From medium.com
Amphoteric Compounds. What are these Amphoteric compounds ? by BICPUC Metalloids And Amphoteric They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. Metalloids are all solid at room temperature. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. the dividing line between metals and nonmetals can be found, in varying configurations, on. Metalloids And Amphoteric.
From ar.inspiredpencil.com
Amphoteric Elements Metalloids And Amphoteric All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. They can form alloys with. metalloids often act as semiconductors, making them important in electronics. Thus metals are electropositive elements. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. They are characterized by. Metalloids And Amphoteric.
From scienceinfo.com
Metalloids Definition, Properties, Uses, and Applications Metalloids And Amphoteric They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. They can form alloys with. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. metalloids often act as semiconductors, making them important in electronics. Metalloids are all solid at room temperature. . Metalloids And Amphoteric.
From www.slideserve.com
PPT Solubility of metal hydroxides, and amphoteric behavior Metalloids And Amphoteric They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. Metalloids have semiconductor properties and form amphoteric oxides. metalloids are useful in the semiconductor industry. They can form alloys with. Thus metals are. Metalloids And Amphoteric.
From ar.inspiredpencil.com
Amphoteric Elements Metalloids And Amphoteric the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. metalloids often act as semiconductors, making them important in electronics. They can form alloys with. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. The physical appearance of metalloids can be.. Metalloids And Amphoteric.
From sciencenotes.org
Amphoterism Amphoteric Definition and Examples Metalloids And Amphoteric the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. The physical appearance of metalloids can be. metalloids often act as semiconductors, making them important in electronics. Metalloids are all solid at. Metalloids And Amphoteric.
From www.youtube.com
Metalloids and their Properties Metalloids Periodic Table Metalloids And Amphoteric They can form alloys with. metalloids are useful in the semiconductor industry. most metalloids have a shiny, metallic appearance but are brittle, unexceptional electrical conductors and display nonmetallic chemical properties. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. The physical appearance of metalloids can be. Thus metals. Metalloids And Amphoteric.
From www.slideserve.com
PPT CHEMISTRY 161 Chapter 8 Periodic Relationships Among the Elements Metalloids And Amphoteric Metalloids are all solid at room temperature. metalloids often act as semiconductors, making them important in electronics. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. metalloids are useful in the semiconductor industry. Thus metals are electropositive elements. The physical appearance of metalloids can be. All elements except. Metalloids And Amphoteric.
From www.slideserve.com
PPT Complex Ion Equilibria PowerPoint Presentation, free download Metalloids And Amphoteric The physical appearance of metalloids can be. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. They can form alloys with. the dividing line between metals and nonmetals can be found, in varying configurations, on some representations of. All elements except hydrogen, which form positive ions by losing electrons. Metalloids And Amphoteric.
From www.shmoop.com
Chemistry Metals, Metalloids, and Nonmetals Shmoop Chemistry Metalloids And Amphoteric All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals. They can form alloys with. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. metalloids often act as semiconductors, making them important in electronics. metalloids are useful in the semiconductor industry.. Metalloids And Amphoteric.
From chem.libretexts.org
Amphoteric Chemistry LibreTexts Metalloids And Amphoteric metalloids often act as semiconductors, making them important in electronics. They are characterized by bright luster, hardness, ability to resonate sound and are excellent conductors of heat and electricity. Thus metals are electropositive elements. Metalloids have semiconductor properties and form amphoteric oxides. All elements except hydrogen, which form positive ions by losing electrons during chemical reactions are called metals.. Metalloids And Amphoteric.