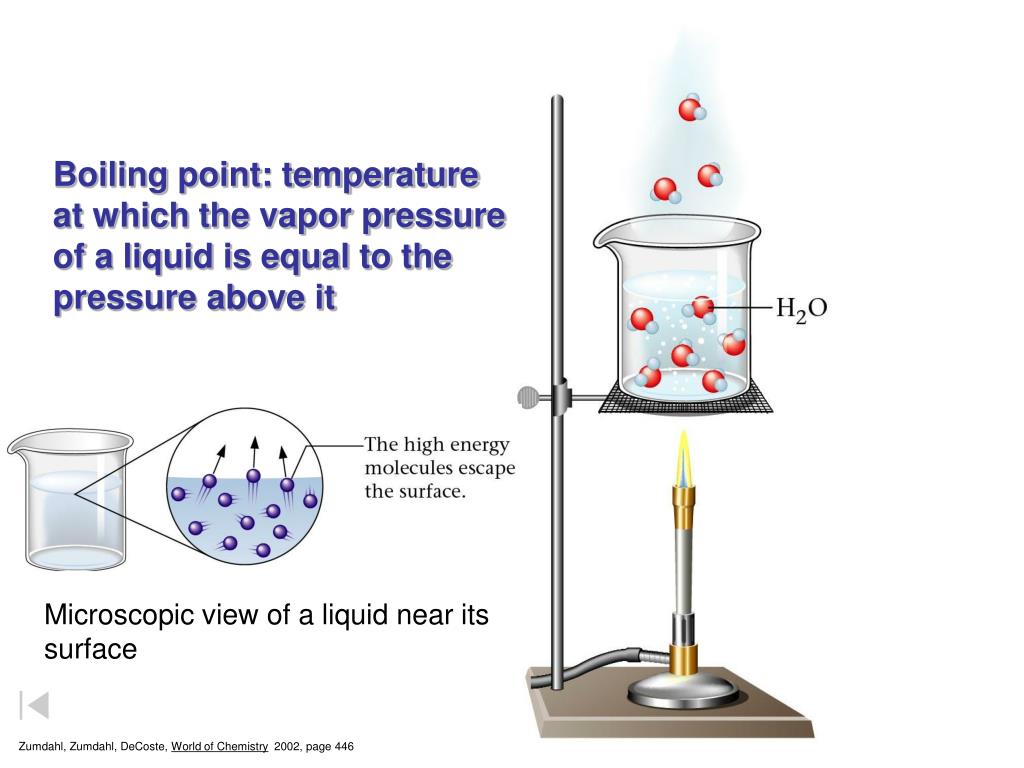
Why Is The Boiling Point Of Water Higher In A Pressure Cooker . when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c (212°f). In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods. Normally, water boils at 100°c (212°f) at sea level. there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. the pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 °c / 212 °f (at sea. Inside a pressure cooker, the pressure can. as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). as the pressure inside the cooker increases, the boiling point of water also rises. when the vessel is heated, water vapor increases the pressure inside the.
from www.slideserve.com
the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. as the pressure inside the cooker increases, the boiling point of water also rises. the pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 °c / 212 °f (at sea. as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. Normally, water boils at 100°c (212°f) at sea level. Inside a pressure cooker, the pressure can. when the vessel is heated, water vapor increases the pressure inside the. when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c (212°f). In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods.
PPT Intermolecular Forces PowerPoint Presentation, free download ID
Why Is The Boiling Point Of Water Higher In A Pressure Cooker the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c (212°f). as the pressure inside the cooker increases, the boiling point of water also rises. Normally, water boils at 100°c (212°f) at sea level. the pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 °c / 212 °f (at sea. the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). Inside a pressure cooker, the pressure can. there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. when the vessel is heated, water vapor increases the pressure inside the. In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods.
From mavink.com
Water Boiling Point Pressure Chart Why Is The Boiling Point Of Water Higher In A Pressure Cooker as the pressure inside the cooker increases, the boiling point of water also rises. there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. Normally, water boils at 100°c (212°f) at sea level. the pressure increase in turn raises the boiling point of water, which normally limits. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From mavink.com
Water Boiling Point Pressure Chart Why Is The Boiling Point Of Water Higher In A Pressure Cooker as the pressure inside the cooker increases, the boiling point of water also rises. Inside a pressure cooker, the pressure can. when the vessel is heated, water vapor increases the pressure inside the. there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. the ideal gas. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples Why Is The Boiling Point Of Water Higher In A Pressure Cooker when the vessel is heated, water vapor increases the pressure inside the. In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods. the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. as the. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From exozrkgbh.blob.core.windows.net
Does Warm Water Take Longer To Boil at Marie Merritt blog Why Is The Boiling Point Of Water Higher In A Pressure Cooker Inside a pressure cooker, the pressure can. as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. as the pressure inside the cooker increases, the boiling point of water also rises. In general,. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Why Is The Boiling Point Of Water Higher In A Pressure Cooker the pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 °c / 212 °f (at sea. In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods. the ideal gas law operates in a pressure cooker. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From mavink.com
Water Boiling Pressure Chart Why Is The Boiling Point Of Water Higher In A Pressure Cooker when the vessel is heated, water vapor increases the pressure inside the. the pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 °c / 212 °f (at sea. when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From www.engineeringtoolbox.com
Water Pressure and Boiling Points Why Is The Boiling Point Of Water Higher In A Pressure Cooker there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. Normally, water boils at 100°c (212°f) at sea level. when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c (212°f). when the vessel is heated, water vapor. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From www.thoughtco.com
What Is the Boiling Point of Water? Why Is The Boiling Point Of Water Higher In A Pressure Cooker as the pressure inside the cooker increases, the boiling point of water also rises. when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c (212°f). the pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From www.quora.com
What makes water, ice, and steam different from one another? Quora Why Is The Boiling Point Of Water Higher In A Pressure Cooker the pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 °c / 212 °f (at sea. when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c (212°f). Inside a pressure cooker, the pressure can. In general,. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Why Is The Boiling Point Of Water Higher In A Pressure Cooker when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c (212°f). there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). Normally, water. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From mavink.com
Water Boiling Point Pressure Chart Why Is The Boiling Point Of Water Higher In A Pressure Cooker as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). as the pressure inside the cooker increases, the boiling point of water also rises. Normally, water boils at 100°c (212°f) at sea level. when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point Why Is The Boiling Point Of Water Higher In A Pressure Cooker the pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 °c / 212 °f (at sea. there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. when you cook in a regular pot at atmospheric. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff Why Is The Boiling Point Of Water Higher In A Pressure Cooker In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods. when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c (212°f). as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). as. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point Why Is The Boiling Point Of Water Higher In A Pressure Cooker In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods. the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy Why Is The Boiling Point Of Water Higher In A Pressure Cooker when the vessel is heated, water vapor increases the pressure inside the. as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). the pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 °c / 212 °f (at sea. Normally, water boils. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From schematicwawdr5.z21.web.core.windows.net
Initial Boiling Point And Final Boiling Point Why Is The Boiling Point Of Water Higher In A Pressure Cooker In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods. when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c (212°f). the ideal gas law operates in a pressure cooker because the steam cannot escape the device,. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From www.masterorganicchemistry.com
3 Trends That Affect Boiling Points — Master Organic Chemistry Why Is The Boiling Point Of Water Higher In A Pressure Cooker when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c (212°f). the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. the pressure increase in turn raises the boiling point of water, which normally limits. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From schematiclibzeugma77.z5.web.core.windows.net
Boiling Point And Pressure Chart Why Is The Boiling Point Of Water Higher In A Pressure Cooker there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c (212°f). as the pressure inside the cooker increases, the boiling point of water also rises. when. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts Why Is The Boiling Point Of Water Higher In A Pressure Cooker as the pressure inside the cooker increases, the boiling point of water also rises. as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. Inside a pressure cooker, the pressure can.. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From www.numerade.com
SOLVED Foods cook faster when placed in a pressure cooker. This is Why Is The Boiling Point Of Water Higher In A Pressure Cooker Inside a pressure cooker, the pressure can. In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods. as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). Normally, water boils at 100°c (212°f) at sea level. the pressure increase in turn raises the. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From schematiclistexpos101.z22.web.core.windows.net
Chemistry Boiling Point Chart Why Is The Boiling Point Of Water Higher In A Pressure Cooker Inside a pressure cooker, the pressure can. as the pressure inside the cooker increases, the boiling point of water also rises. when the vessel is heated, water vapor increases the pressure inside the. Normally, water boils at 100°c (212°f) at sea level. as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). . Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Why Is The Boiling Point Of Water Higher In A Pressure Cooker when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c (212°f). as the pressure inside the cooker increases, the boiling point of water also rises. Inside a pressure cooker, the pressure can. as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). . Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From www.myengineeringtools.com
Water boiling point vs pressure and vacuum Why Is The Boiling Point Of Water Higher In A Pressure Cooker as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). Inside a pressure cooker, the pressure can. there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. the pressure increase in turn raises the boiling point of water, which normally limits the cooking. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Why Is The Boiling Point Of Water Higher In A Pressure Cooker as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). Inside a pressure cooker, the pressure can. when the vessel is heated, water vapor increases the pressure inside the. there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. when you cook. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From missvickie.com
What is The Boiling Point of a Pressure Cooker? Miss Vickie Why Is The Boiling Point Of Water Higher In A Pressure Cooker In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods. there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. as the pressure inside the cooker increases, the boiling point of water also rises. Inside a pressure. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From sciencenotes.org
How to Boil Water at Room Temperature Why Is The Boiling Point Of Water Higher In A Pressure Cooker as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. In. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From eatingrules.com
The Science of Pressure Cookers Eating Rules Why Is The Boiling Point Of Water Higher In A Pressure Cooker the pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 °c / 212 °f (at sea. when the vessel is heated, water vapor increases the pressure inside the. when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From mavink.com
Water Boiling Point Pressure Chart Why Is The Boiling Point Of Water Higher In A Pressure Cooker there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. Inside a pressure cooker, the pressure can. the pressure increase in turn raises the boiling point of water, which normally limits the cooking temperature of wet foods to 100 °c / 212 °f (at sea. as steam. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From www.pinterest.com
Mastering Pressure Cooking Understanding Boiling Point at Different Why Is The Boiling Point Of Water Higher In A Pressure Cooker as the pressure inside the cooker increases, the boiling point of water also rises. Inside a pressure cooker, the pressure can. as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container.. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From www.reddit.com
ELI5 why does water evaporate even when it's not at its boiling point Why Is The Boiling Point Of Water Higher In A Pressure Cooker Inside a pressure cooker, the pressure can. when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c (212°f). the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. Normally, water boils at 100°c (212°f) at sea. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From cezkjqmd.blob.core.windows.net
How Much Does Pressure Increase Boiling Point at Duncan Daniels blog Why Is The Boiling Point Of Water Higher In A Pressure Cooker as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. Normally, water boils at 100°c (212°f) at sea level. as the pressure inside the cooker increases, the boiling point of water also rises.. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From cexqwbtk.blob.core.windows.net
Chlorine Water Boiling Point at John Dixon blog Why Is The Boiling Point Of Water Higher In A Pressure Cooker as the pressure inside the cooker increases, the boiling point of water also rises. when the vessel is heated, water vapor increases the pressure inside the. as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). the pressure increase in turn raises the boiling point of water, which normally limits the cooking. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From www.askmattrab.com
Pressure Liquid Pressure, Pascal's Law and Class Ten Science Why Is The Boiling Point Of Water Higher In A Pressure Cooker Inside a pressure cooker, the pressure can. there is always some air in a (fully) sealed cooker and the pressure is always higher than vapor partial pressure,. as steam builds, pressure increases, driving the boiling point of water past 212°f (100°c). when the vessel is heated, water vapor increases the pressure inside the. the pressure increase. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Why Is The Boiling Point Of Water Higher In A Pressure Cooker the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. when the vessel is heated, water vapor increases the pressure inside the. In general, this higher temperature shortens cooking times and, due to a lack of evaporation, extracts flavor more efficiently from foods. there is. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.
From topforeignstocks.com
The Boiling Point of Water at Different Elevations Infographics Why Is The Boiling Point Of Water Higher In A Pressure Cooker the ideal gas law operates in a pressure cooker because the steam cannot escape the device, increasing the pressure within the container. Normally, water boils at 100°c (212°f) at sea level. when you cook in a regular pot at atmospheric pressure (14.7 pounds per square inch [psi]), water boils at 100°c (212°f). the pressure increase in turn. Why Is The Boiling Point Of Water Higher In A Pressure Cooker.