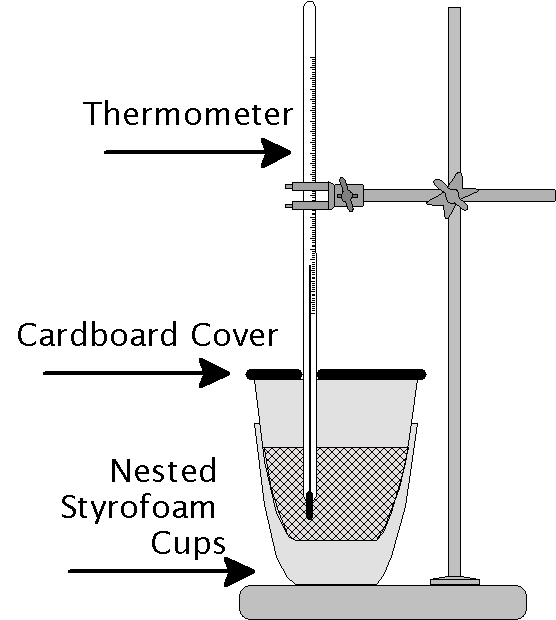
Characteristics Of Calorimeter In Physics . Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Most students likely do not remember using such a fancy piece of equipment known as a. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. The most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro calorimeters, and accelerated rate calorimeters. A calorimeter is a device used to measure the quantity of heat transferred to or from an object. The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. A calorimeter functions by isolating the system undergoing a reaction from its surroundings to accurately measure the heat exchanged. A calorimeter is a device that is in use for measuring the warmth of chemical reactions or physical changes also as heat capacity. Calorimetry is used to measure amounts of heat transferred to or from a substance.
from kaffee.50webs.com
Calorimetry is used to measure amounts of heat transferred to or from a substance. The most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro calorimeters, and accelerated rate calorimeters. A calorimeter is a device used to measure the quantity of heat transferred to or from an object. A calorimeter functions by isolating the system undergoing a reaction from its surroundings to accurately measure the heat exchanged. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. To do so, the heat is exchanged with a calibrated object (calorimeter). A calorimeter is a device that is in use for measuring the warmth of chemical reactions or physical changes also as heat capacity. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials.
Lab Calorimetry
Characteristics Of Calorimeter In Physics Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter functions by isolating the system undergoing a reaction from its surroundings to accurately measure the heat exchanged. Most students likely do not remember using such a fancy piece of equipment known as a. To do so, the heat is exchanged with a calibrated object (calorimeter). A calorimeter is a device that is in use for measuring the warmth of chemical reactions or physical changes also as heat capacity. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro calorimeters, and accelerated rate calorimeters. A calorimeter is a device used to measure the quantity of heat transferred to or from an object.
From www.science-revision.co.uk
Calorimetry Characteristics Of Calorimeter In Physics Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. The most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro calorimeters, and accelerated. Characteristics Of Calorimeter In Physics.
From www.thoughtco.com
Calorimeter Definition in Chemistry Characteristics Of Calorimeter In Physics A calorimeter is a device that is in use for measuring the warmth of chemical reactions or physical changes also as heat capacity. A calorimeter is a device used to measure the quantity of heat transferred to or from an object. The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process. Characteristics Of Calorimeter In Physics.
From 2012books.lardbucket.org
Calorimetry Characteristics Of Calorimeter In Physics The most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro calorimeters, and accelerated rate calorimeters. To do so, the heat is exchanged with a calibrated object (calorimeter). Most students likely do not remember using such a fancy piece of equipment known as a. The temperature change measured by the calorimeter is used to derive the amount. Characteristics Of Calorimeter In Physics.
From kaffee.50webs.com
Lab Calorimetry Characteristics Of Calorimeter In Physics Most students likely do not remember using such a fancy piece of equipment known as a. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. The temperature change measured by the calorimeter is used to derive the amount of. Characteristics Of Calorimeter In Physics.
From www.youtube.com
Numericals of Calorimetry Calorimetry Class 10 Physics ICSE YouTube Characteristics Of Calorimeter In Physics The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. Most students likely do not remember using such a fancy piece of equipment known as a. A calorimeter functions by isolating the system undergoing a reaction from its surroundings to accurately measure the heat exchanged. A calorimeter is a. Characteristics Of Calorimeter In Physics.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Characteristics Of Calorimeter In Physics Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. A calorimeter functions by isolating the system undergoing a reaction from its surroundings to accurately. Characteristics Of Calorimeter In Physics.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Characteristics Of Calorimeter In Physics A calorimeter functions by isolating the system undergoing a reaction from its surroundings to accurately measure the heat exchanged. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. To do so, the heat is exchanged with a calibrated object (calorimeter). The temperature change measured by the calorimeter. Characteristics Of Calorimeter In Physics.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Characteristics Of Calorimeter In Physics A calorimeter is a device that is in use for measuring the warmth of chemical reactions or physical changes also as heat capacity. A calorimeter is a device used to measure the quantity of heat transferred to or from an object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a branch of. Characteristics Of Calorimeter In Physics.
From 2012books.lardbucket.org
Calorimetry Characteristics Of Calorimeter In Physics A calorimeter functions by isolating the system undergoing a reaction from its surroundings to accurately measure the heat exchanged. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. To do so, the heat is exchanged with a calibrated object. Characteristics Of Calorimeter In Physics.
From byjus.com
State the Principle of calorimetry Characteristics Of Calorimeter In Physics A calorimeter is a device that is in use for measuring the warmth of chemical reactions or physical changes also as heat capacity. Calorimetry is used to measure amounts of heat transferred to or from a substance. The most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro calorimeters, and accelerated rate calorimeters. A calorimeter functions by. Characteristics Of Calorimeter In Physics.
From www.youtube.com
Calorimetry Lecture 4 Thermal Physics Explanation of Temperature Time Characteristics Of Calorimeter In Physics The most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro calorimeters, and accelerated rate calorimeters. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to measure the quantity of heat transferred to or from an object. A container that prevents heat transfer in or out. Characteristics Of Calorimeter In Physics.
From scienceinfo.com
Calorimeter Definition, Types and Uses Characteristics Of Calorimeter In Physics A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. The most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro calorimeters, and accelerated rate calorimeters. Calorimeter, device for measuring the heat developed during a mechanical, electrical,. Characteristics Of Calorimeter In Physics.
From www.slideserve.com
PPT Purpose of the Experiment PowerPoint Presentation, free download Characteristics Of Calorimeter In Physics The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. Most students likely do not remember using such a fancy piece of equipment known as a. Calorimetry is used to measure amounts of heat transferred to or from a substance. A container that prevents heat transfer in or out. Characteristics Of Calorimeter In Physics.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Characteristics Of Calorimeter In Physics The most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro calorimeters, and accelerated rate calorimeters. The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device that. Characteristics Of Calorimeter In Physics.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Characteristics Of Calorimeter In Physics Calorimetry is used to measure amounts of heat transferred to or from a substance. The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. A calorimeter is a device that is in use for measuring the warmth of chemical reactions or physical changes also as heat capacity. A container. Characteristics Of Calorimeter In Physics.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Characteristics Of Calorimeter In Physics The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. To do so, the heat is exchanged with a calibrated object (calorimeter). A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific. Characteristics Of Calorimeter In Physics.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube Characteristics Of Calorimeter In Physics Calorimetry is used to measure amounts of heat transferred to or from a substance. The most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro calorimeters, and accelerated rate calorimeters. The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. A container that prevents heat transfer. Characteristics Of Calorimeter In Physics.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Characteristics Of Calorimeter In Physics To do so, the heat is exchanged with a calibrated object (calorimeter). The most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro calorimeters, and accelerated rate calorimeters. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The temperature change measured by the calorimeter. Characteristics Of Calorimeter In Physics.
From www.youtube.com
Principle of Calorimetry YouTube Characteristics Of Calorimeter In Physics The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. To do so, the heat is exchanged with a. Characteristics Of Calorimeter In Physics.
From www.animalia-life.club
Calorimeter Diagram Characteristics Of Calorimeter In Physics To do so, the heat is exchanged with a calibrated object (calorimeter). Most students likely do not remember using such a fancy piece of equipment known as a. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device used to measure. Characteristics Of Calorimeter In Physics.
From app.jove.com
Calorimetry Concept Physics JoVe Characteristics Of Calorimeter In Physics Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Most students likely do not remember using such a fancy piece of equipment known as a. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimeter, device for measuring the heat developed during. Characteristics Of Calorimeter In Physics.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Characteristics Of Calorimeter In Physics Most students likely do not remember using such a fancy piece of equipment known as a. The most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro calorimeters, and accelerated rate calorimeters. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter functions by isolating the system undergoing a reaction from. Characteristics Of Calorimeter In Physics.
From study.com
Calorimetry Definition, Equation & Types Lesson Characteristics Of Calorimeter In Physics To do so, the heat is exchanged with a calibrated object (calorimeter). Most students likely do not remember using such a fancy piece of equipment known as a. A calorimeter is a device that is in use for measuring the warmth of chemical reactions or physical changes also as heat capacity. The temperature change measured by the calorimeter is used. Characteristics Of Calorimeter In Physics.
From slideplayer.com
Calorimetry Chapter ppt download Characteristics Of Calorimeter In Physics Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to measure the quantity of heat transferred to or from an object. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The most common types of calorimeters. Characteristics Of Calorimeter In Physics.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Characteristics Of Calorimeter In Physics A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A calorimeter is a device used to measure. Characteristics Of Calorimeter In Physics.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Characteristics Of Calorimeter In Physics A calorimeter functions by isolating the system undergoing a reaction from its surroundings to accurately measure the heat exchanged. A calorimeter is a device that is in use for measuring the warmth of chemical reactions or physical changes also as heat capacity. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a. Characteristics Of Calorimeter In Physics.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Characteristics Of Calorimeter In Physics Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. A calorimeter is a device that is in use for measuring the warmth of. Characteristics Of Calorimeter In Physics.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Characteristics Of Calorimeter In Physics Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter is a device used to measure the quantity of heat transferred to or from an object. A calorimeter is a device that is in use for measuring the warmth of chemical reactions or physical changes also as heat capacity. Calorimeter, device for measuring the. Characteristics Of Calorimeter In Physics.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Characteristics Of Calorimeter In Physics A calorimeter is a device that is in use for measuring the warmth of chemical reactions or physical changes also as heat capacity. Most students likely do not remember using such a fancy piece of equipment known as a. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make. Characteristics Of Calorimeter In Physics.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Characteristics Of Calorimeter In Physics To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. Characteristics Of Calorimeter In Physics.
From ar.inspiredpencil.com
Calorimeter Diagram Characteristics Of Calorimeter In Physics The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. A calorimeter is a device used to measure the quantity of heat transferred to or from an object. A calorimeter is a device that is in use for measuring the warmth of chemical reactions or physical changes also as. Characteristics Of Calorimeter In Physics.
From www.youtube.com
Learn about Calorimeter/ Calorimetry/Physics(Heat) 🔥 YouTube Characteristics Of Calorimeter In Physics The most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro calorimeters, and accelerated rate calorimeters. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. Calorimetry is a branch of science concerned with measuring a body’s. Characteristics Of Calorimeter In Physics.
From louis.pressbooks.pub
Calorimetry (9.2) General Chemistry Characteristics Of Calorimeter In Physics The most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro calorimeters, and accelerated rate calorimeters. The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity. Characteristics Of Calorimeter In Physics.
From physique.ensc-rennes.fr
TP calorimetry Characteristics Of Calorimeter In Physics The temperature change measured by the calorimeter is used to derive the amount of heat transferred by the process under study. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. To do so,. Characteristics Of Calorimeter In Physics.
From www.slideserve.com
PPT An introduction to calorimeters for particle physics PowerPoint Characteristics Of Calorimeter In Physics A calorimeter is a device used to measure the quantity of heat transferred to or from an object. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts. Characteristics Of Calorimeter In Physics.