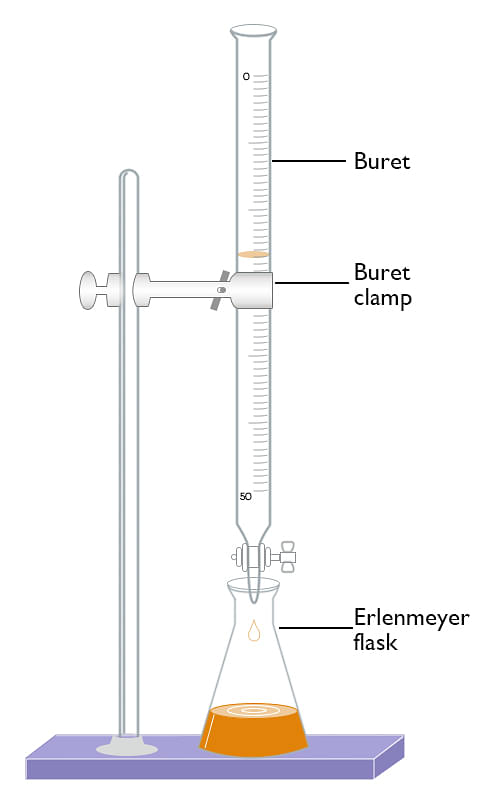
Titration In Chemistry Example . By this process, the acid or. A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4) weighing 0.3421 g is dissolved in distilled water. Perform and interpret titration calculations. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In this comprehensive guide, we’ll take. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. In this topic, we will certainly explain the. Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration.
from collegedunia.com
In this comprehensive guide, we’ll take. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4) weighing 0.3421 g is dissolved in distilled water. Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. By this process, the acid or. Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In this topic, we will certainly explain the.
Volumetric Analysis Titration, Types, Principle & Procedure
Titration In Chemistry Example In this topic, we will certainly explain the. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. In this topic, we will certainly explain the. In this comprehensive guide, we’ll take. By this process, the acid or. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Perform and interpret titration calculations. A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4) weighing 0.3421 g is dissolved in distilled water. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration. Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Titration In Chemistry Example A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration. Perform and interpret titration calculations. A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4) weighing 0.3421 g is dissolved. Titration In Chemistry Example.
From www.dreamstime.com
Analytical Chemistry Titration Equipment. Laboratory Glassware in a Titration In Chemistry Example A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In this topic, we will certainly explain the. Perform and interpret titration calculations. Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration. In this comprehensive guide, we’ll take. Titration is the slow. Titration In Chemistry Example.
From joizlgxlm.blob.core.windows.net
Titration Experiments In Supramolecular Chemistry at Larry Ferrill blog Titration In Chemistry Example Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A sample of pure potassium hydrogen phthalate. Titration In Chemistry Example.
From dxobyswdg.blob.core.windows.net
Titration Means What In Chemistry at Sabrina Reyes blog Titration In Chemistry Example In this topic, we will certainly explain the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In this comprehensive guide, we’ll take. Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration. Titration In Chemistry Example.
From www.scribd.com
Titration (Chemistry Experiment Report) PDF Titration Chemistry Titration In Chemistry Example Perform and interpret titration calculations. In this comprehensive guide, we’ll take. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In this topic, we will. Titration In Chemistry Example.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration In Chemistry Example A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4) weighing 0.3421 g is dissolved in distilled water. By this process, the acid or. Perform and interpret titration calculations. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration. Titration In Chemistry Example.
From facts.net
8 Captivating Facts About AcidBase Titration Titration In Chemistry Example A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a. Titration In Chemistry Example.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration In Chemistry Example A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4) weighing 0.3421 g is dissolved in distilled water. Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration. In this topic, we will certainly explain the. Perform and interpret titration calculations. A titration is a laboratory technique used to precisely. Titration In Chemistry Example.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration In Chemistry Example Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using. Titration In Chemistry Example.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE Titration In Chemistry Example A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4). Titration In Chemistry Example.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration In Chemistry Example A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4) weighing 0.3421 g is dissolved in distilled water. Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. By this process, the acid or. In this comprehensive guide, we’ll take. A. Titration In Chemistry Example.
From www.flinnsci.com
360 Science Titrations and the Study of AcidBase Chemistry Titration In Chemistry Example Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is a quantitative analysis to determine. Titration In Chemistry Example.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration In Chemistry Example Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration. Perform and interpret titration calculations. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration refers to a process where the use of a solution of. Titration In Chemistry Example.
From chem.libretexts.org
AcidBase Titrations Chemistry LibreTexts Titration In Chemistry Example Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In this topic, we will certainly explain the. A titration. Titration In Chemistry Example.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration In Chemistry Example In this comprehensive guide, we’ll take. Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration. Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. Perform and interpret titration calculations. A sample of pure potassium hydrogen. Titration In Chemistry Example.
From joieldmit.blob.core.windows.net
How Is Titration Used In Medicine at Shelia Wiley blog Titration In Chemistry Example In this comprehensive guide, we’ll take. In this topic, we will certainly explain the. Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. By this. Titration In Chemistry Example.
From tagvault.org
Standardization vs Titration in Chemistry (Explained) Titration In Chemistry Example Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4) weighing 0.3421 g is dissolved in distilled water. Titration refers to a process where the use of a solution of known concentration takes. Titration In Chemistry Example.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration In Chemistry Example In this topic, we will certainly explain the. Perform and interpret titration calculations. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In this comprehensive guide, we’ll take. Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration.. Titration In Chemistry Example.
From www.science-revision.co.uk
Titrations Titration In Chemistry Example Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration. By this process, the acid or. Titration is a quantitative analysis to determine the concentration of an. Titration In Chemistry Example.
From theedge.com.hk
Chemistry How To Titration The Edge Titration In Chemistry Example Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. In this comprehensive guide, we’ll take. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a. Titration In Chemistry Example.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration In Chemistry Example Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. Perform and interpret titration calculations. In this topic, we will certainly explain the. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. Titration In Chemistry Example.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titration In Chemistry Example Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4) weighing 0.3421 g is dissolved in distilled water. A titration is a laboratory technique used to precisely measure molar concentration of. Titration In Chemistry Example.
From quizizz.com
Titration Method Chemistry Quiz Quizizz Titration In Chemistry Example A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4) weighing 0.3421 g is dissolved in distilled water. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. By this process, the acid or. In this comprehensive guide, we’ll take.. Titration In Chemistry Example.
From www.youtube.com
TYPES OF TITRATION IN CHEMISTRY ACID BASE TITRATION REDOX TITRATION Titration In Chemistry Example In this topic, we will certainly explain the. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. By this process, the acid or. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. Titration In Chemistry Example.
From joiyfxbtq.blob.core.windows.net
Titration Method Image at Jodie Massey blog Titration In Chemistry Example Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a solution of known concentration in a drop at a time. Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration. In this comprehensive guide, we’ll take. Titration is the slow addition of one solution of a known. Titration In Chemistry Example.
From collegedunia.com
Volumetric Analysis Titration, Types, Principle & Procedure Titration In Chemistry Example In this comprehensive guide, we’ll take. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4) weighing 0.3421 g is dissolved in distilled water. Titration is the slow addition of. Titration In Chemistry Example.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration In Chemistry Example Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. In this comprehensive guide, we’ll take. A titration is. Titration In Chemistry Example.
From www.scribd.com
Titration PDF Titration Chemistry Titration In Chemistry Example By this process, the acid or. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration. In this topic, we will certainly explain the. A sample of pure potassium hydrogen phthalate (khc 8. Titration In Chemistry Example.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration In Chemistry Example Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. In this topic, we will certainly explain the. Titration is a chemical process that involves mixing solutions and carefully measuring their reactions to calculate concentration. Perform and interpret titration calculations. In this comprehensive. Titration In Chemistry Example.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration In Chemistry Example By this process, the acid or. A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4) weighing 0.3421 g is dissolved in distilled water. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Perform and interpret titration calculations. Titration is a chemical. Titration In Chemistry Example.
From celtjcvb.blob.core.windows.net
Titration In Simple Terms at John Haslett blog Titration In Chemistry Example Titration refers to a process where the use of a solution of known concentration takes place for the determination of the concentration of an unknown solution. By this process, the acid or. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration. Titration In Chemistry Example.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration In Chemistry Example Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In this topic, we will certainly explain the. A sample of pure potassium. Titration In Chemistry Example.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration In Chemistry Example A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. By this process, the acid or. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titration is the slow addition of one. Titration In Chemistry Example.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration In Chemistry Example A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4) weighing 0.3421 g is dissolved in distilled water. By this process, the acid or. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. In this comprehensive guide, we’ll take.. Titration In Chemistry Example.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Titration In Chemistry Example Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. A sample of pure potassium hydrogen phthalate (khc 8 h 4 o 4) weighing 0.3421 g is dissolved in distilled water. In this comprehensive guide, we’ll take. Titration is a chemical process that. Titration In Chemistry Example.