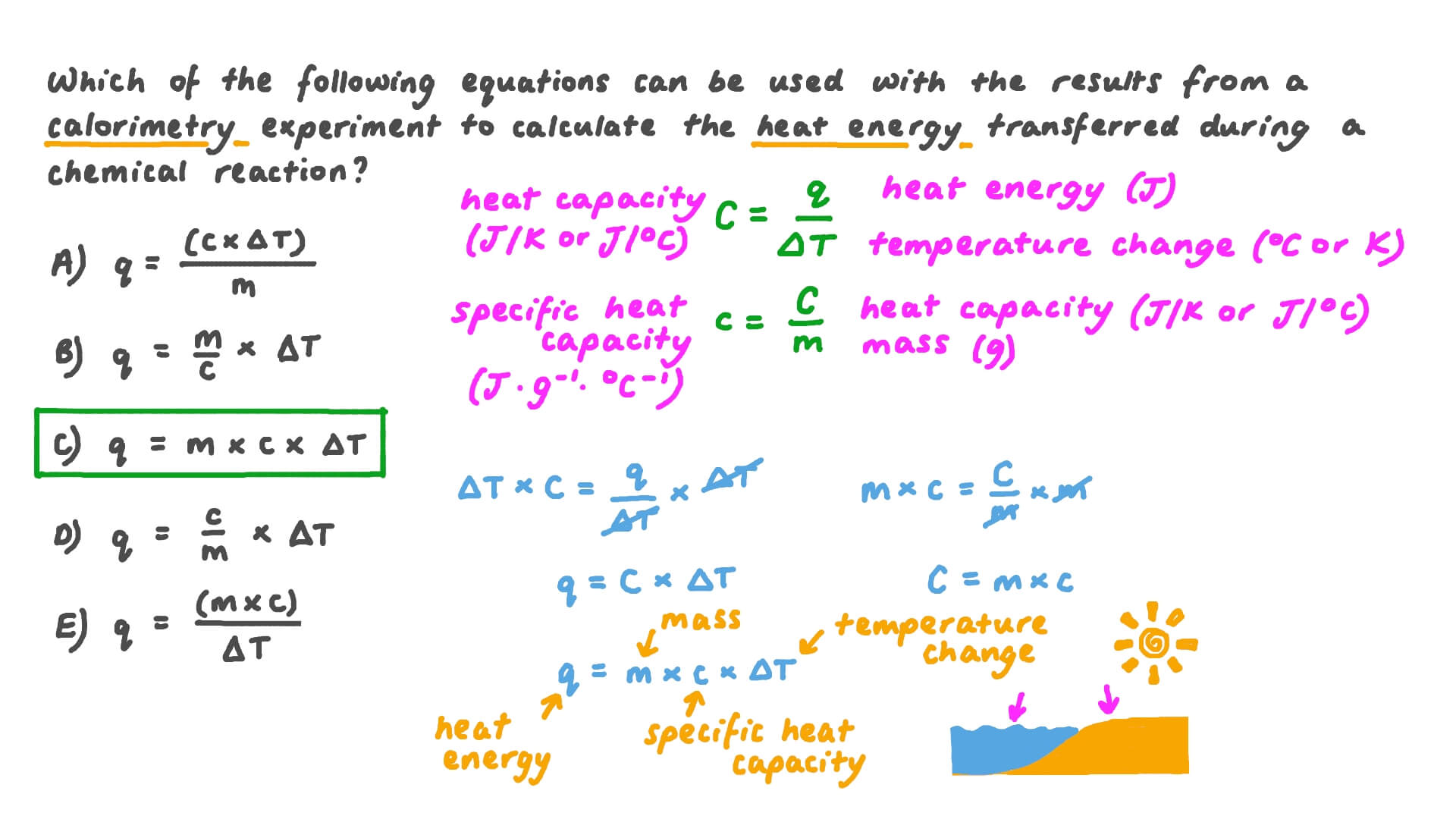Calorimeter Equation Specific Heat . Calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at 22.0°c and the resulting temperature of the mixture. The specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p) is the. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). If the amount of heat absorbed by a calorimeter is too large to neglect or if we require more accurate results, then we must take into account the. We see from this table that the specific heat of water is five times that of glass and 10 times that of iron, which. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat capacity (c) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. Where q is the energy added and δt is the change in temperature. Table 1.3 lists representative values of specific heat for various substances.
from www.gbu-presnenskij.ru
The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p) is the. Where q is the energy added and δt is the change in temperature. If the amount of heat absorbed by a calorimeter is too large to neglect or if we require more accurate results, then we must take into account the. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at 22.0°c and the resulting temperature of the mixture. Table 1.3 lists representative values of specific heat for various substances. We see from this table that the specific heat of water is five times that of glass and 10 times that of iron, which. The specific heat capacity (c) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1.
Chapter 25 Essential Calorimetry Formulae James Kennedy, 47 OFF
Calorimeter Equation Specific Heat The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Where q is the energy added and δt is the change in temperature. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). If the amount of heat absorbed by a calorimeter is too large to neglect or if we require more accurate results, then we must take into account the. The specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p) is the. Table 1.3 lists representative values of specific heat for various substances. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at 22.0°c and the resulting temperature of the mixture. We see from this table that the specific heat of water is five times that of glass and 10 times that of iron, which. The specific heat capacity (c) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1.
From www.slideserve.com
PPT HEAT EQUATION (in Table T) PowerPoint Presentation, free download Calorimeter Equation Specific Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p) is the. Table 1.3 lists representative values. Calorimeter Equation Specific Heat.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimeter Equation Specific Heat Table 1.3 lists representative values of specific heat for various substances. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The specific heat capacity (c) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance. Calorimeter Equation Specific Heat.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Equation Specific Heat The specific heat capacity (c) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. Calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at 22.0°c and the resulting temperature of the. Calorimeter Equation Specific Heat.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6133898 Calorimeter Equation Specific Heat We see from this table that the specific heat of water is five times that of glass and 10 times that of iron, which. Table 1.3 lists representative values of specific heat for various substances. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Where q is the energy. Calorimeter Equation Specific Heat.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimeter Equation Specific Heat If the amount of heat absorbed by a calorimeter is too large to neglect or if we require more accurate results, then we must take into account the. Calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at 22.0°c and the resulting temperature of the mixture. The formula. Calorimeter Equation Specific Heat.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Calorimeter Equation Specific Heat The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p) is the. If the amount of heat. Calorimeter Equation Specific Heat.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimeter Equation Specific Heat We see from this table that the specific heat of water is five times that of glass and 10 times that of iron, which. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Table 1.3 lists representative values of specific heat for various substances. The formula for specific heat. Calorimeter Equation Specific Heat.
From worksheetdbsofar.z13.web.core.windows.net
How To Calculate Calorimetry Problems Calorimeter Equation Specific Heat Calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at 22.0°c and the resulting temperature of the mixture. Table 1.3 lists representative values of specific heat for various substances. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m ×. Calorimeter Equation Specific Heat.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimeter Equation Specific Heat The specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p) is the. If the amount of heat absorbed by a calorimeter is too large to neglect or if we require more accurate results, then we must take. Calorimeter Equation Specific Heat.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Equation Specific Heat We see from this table that the specific heat of water is five times that of glass and 10 times that of iron, which. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on. Calorimeter Equation Specific Heat.
From revisionug.com
What is Calorimetry? Calorimeter Equation Specific Heat Table 1.3 lists representative values of specific heat for various substances. The specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p) is the. The symbol c stands for the specific heat (also called “specific heat capacity”) and. Calorimeter Equation Specific Heat.
From worksheetdbroyces.z21.web.core.windows.net
Coffee Cup Calorimetry Calculator Calorimeter Equation Specific Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p) is the. Where q is the energy. Calorimeter Equation Specific Heat.
From www.youtube.com
Specific Heat Equation Stated Clearly YouTube Calorimeter Equation Specific Heat We see from this table that the specific heat of water is five times that of glass and 10 times that of iron, which. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The specific heat capacity (c) of a substance, commonly called its specific heat, is the quantity. Calorimeter Equation Specific Heat.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Calorimeter Equation Specific Heat The specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p) is the. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat capacity (c). Calorimeter Equation Specific Heat.
From www.numerade.com
SOLVED Define the following and show their relative equations and Calorimeter Equation Specific Heat Calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at 22.0°c and the resulting temperature of the mixture. The specific heat capacity (c) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by. Calorimeter Equation Specific Heat.
From ar.inspiredpencil.com
Calculate Thermal Energy Calorimeter Equation Specific Heat The specific heat capacity (c) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. If the amount of heat absorbed by a calorimeter is too large to neglect or if we require more accurate results, then we must take into account the. We. Calorimeter Equation Specific Heat.
From studylib.net
Heat Capacity of Metals PreLab Calorimeter Equation Specific Heat The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). If the amount of heat absorbed by a calorimeter is too large to neglect or if we require more accurate results, then we must take into account the. The symbol c stands for the specific heat (also called “specific heat. Calorimeter Equation Specific Heat.
From www.gbu-presnenskij.ru
Chapter 25 Essential Calorimetry Formulae James Kennedy, 47 OFF Calorimeter Equation Specific Heat Table 1.3 lists representative values of specific heat for various substances. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. If the amount of heat absorbed by a calorimeter. Calorimeter Equation Specific Heat.
From www.slideserve.com
PPT Specific Heat PowerPoint Presentation, free download ID3721637 Calorimeter Equation Specific Heat We see from this table that the specific heat of water is five times that of glass and 10 times that of iron, which. Calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at 22.0°c and the resulting temperature of the mixture. The specific heat (c s) of. Calorimeter Equation Specific Heat.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimeter Equation Specific Heat The specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p) is the. If the amount of heat absorbed by a calorimeter is too large to neglect or if we require more accurate results, then we must take. Calorimeter Equation Specific Heat.
From www.youtube.com
Calorimetry Part 2 calculations YouTube Calorimeter Equation Specific Heat If the amount of heat absorbed by a calorimeter is too large to neglect or if we require more accurate results, then we must take into account the. Table 1.3 lists representative values of specific heat for various substances. The specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g. Calorimeter Equation Specific Heat.
From www.youtube.com
Thermodynamics SPECIFIC HEATS cv & cp in 12 Minutes! YouTube Calorimeter Equation Specific Heat The specific heat capacity (c) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. Calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at 22.0°c and the resulting temperature of the. Calorimeter Equation Specific Heat.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6655927 Calorimeter Equation Specific Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat capacity (c p) is the. The specific heat capacity (c). Calorimeter Equation Specific Heat.
From www.youtube.com
Calorimetry Calculate Specific Heat YouTube Calorimeter Equation Specific Heat Table 1.3 lists representative values of specific heat for various substances. Calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at 22.0°c and the resulting temperature of the mixture. We see from this table that the specific heat of water is five times that of glass and 10. Calorimeter Equation Specific Heat.
From www.youtube.com
Calorimetry calculation YouTube Calorimeter Equation Specific Heat The specific heat capacity (c) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Calculate the calorimeter constant if 150.0 g of lead. Calorimeter Equation Specific Heat.
From quiztricksters.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter Equation Specific Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat capacity (c) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. The specific heat (c s) of a substance is. Calorimeter Equation Specific Heat.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimeter Equation Specific Heat We see from this table that the specific heat of water is five times that of glass and 10 times that of iron, which. If the amount of heat absorbed by a calorimeter is too large to neglect or if we require more accurate results, then we must take into account the. The formula for specific heat capacity, c, of. Calorimeter Equation Specific Heat.
From quizzlibhofmann.z19.web.core.windows.net
Specific Heat Calculation Examples Calorimeter Equation Specific Heat If the amount of heat absorbed by a calorimeter is too large to neglect or if we require more accurate results, then we must take into account the. The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Where q is the energy added and δt is the change in. Calorimeter Equation Specific Heat.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Equation Specific Heat The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). If the amount of heat absorbed by a calorimeter is too large to neglect or if we require more accurate results, then we must take into account the. The specific heat (c s) of a substance is the amount of. Calorimeter Equation Specific Heat.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Equation Specific Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Table 1.3 lists representative values of specific heat for various substances. Where q is the energy added and δt is the change in temperature. The formula for specific heat capacity, c, of a substance with mass m, is c =. Calorimeter Equation Specific Heat.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Calorimeter Equation Specific Heat Calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at 22.0°c and the resulting temperature of the mixture. The specific heat (c s) of a substance is the amount of energy needed to raise the temperature of 1 g of the substance by 1°c, and the molar heat. Calorimeter Equation Specific Heat.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Calorimeter Equation Specific Heat The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat capacity (c) of a substance, commonly called its specific heat, is the quantity of heat required to raise the temperature of 1 gram of a substance by 1. If the amount of heat absorbed by a calorimeter. Calorimeter Equation Specific Heat.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry Calorimeter Equation Specific Heat Where q is the energy added and δt is the change in temperature. Table 1.3 lists representative values of specific heat for various substances. Calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at 22.0°c and the resulting temperature of the mixture. The specific heat capacity (c) of. Calorimeter Equation Specific Heat.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Calorimeter Equation Specific Heat The formula for specific heat capacity, c, of a substance with mass m, is c = q /(m × δt). Table 1.3 lists representative values of specific heat for various substances. If the amount of heat absorbed by a calorimeter is too large to neglect or if we require more accurate results, then we must take into account the. Calculate. Calorimeter Equation Specific Heat.
From www.youtube.com
Measurement and effects of heat (part 3)! Class 8 Specific heat Calorimeter Equation Specific Heat Calculate the calorimeter constant if 150.0 g of lead at 100.0°c were placed in a calorimeter with 50.0 g of water at 22.0°c and the resulting temperature of the mixture. Where q is the energy added and δt is the change in temperature. The specific heat capacity (c) of a substance, commonly called its specific heat, is the quantity of. Calorimeter Equation Specific Heat.