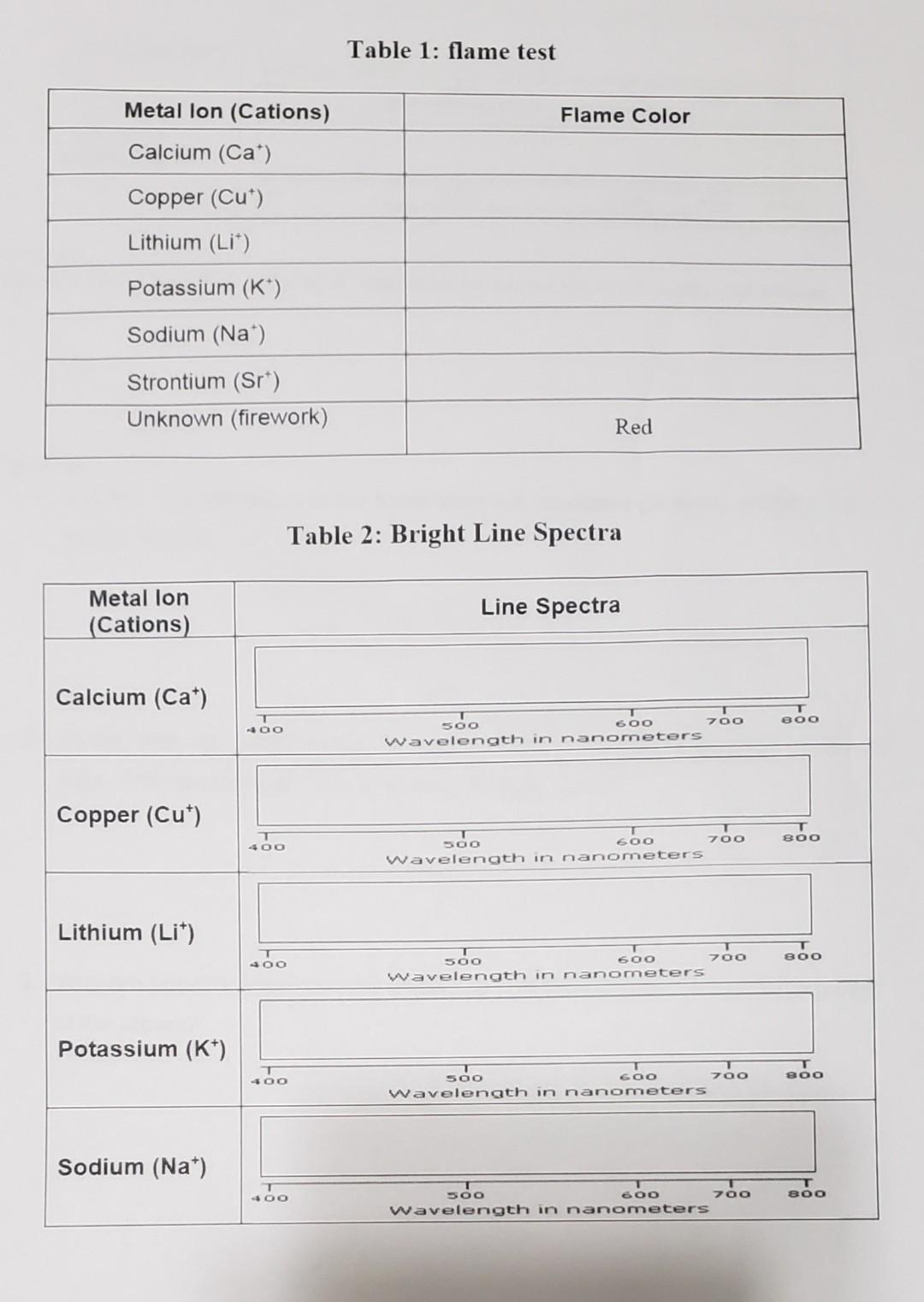
Flame Test & Spectroscopy Answer Key . Test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and to identify the. What metal ion causes fireworks to be red? Mostly the flame test detects. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and. Observe the flame test for seven different metallic ions. 6m hydrochloric acid pour 150. The objectives of this lab are to: When metal ion samples are heated in a flame test,. Study with quizlet and memorize flashcards containing terms like what is a flame test? Flame tests and spectroscopy can be used to identify metal ions based on the unique colors they emit. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and. Use a flame test to determine which ion salt produces the red. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. How is it done?, under what. Identify an unknown metallic element, using the flame test.
from www.chegg.com
Test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and to identify the. Identify an unknown metallic element, using the flame test. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and. What metal ion causes fireworks to be red? When metal ion samples are heated in a flame test,. The objectives of this lab are to: Use a flame test to determine which ion salt produces the red. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. Flame tests and spectroscopy can be used to identify metal ions based on the unique colors they emit. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and.
Solved Flame Test & Spectroscopy Background Electrons are
Flame Test & Spectroscopy Answer Key Mostly the flame test detects. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and. When metal ion samples are heated in a flame test,. Study with quizlet and memorize flashcards containing terms like what is a flame test? Observe the flame test for seven different metallic ions. Identify an unknown metallic element, using the flame test. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and. The objectives of this lab are to: Use a flame test to determine which ion salt produces the red. Test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and to identify the. 6m hydrochloric acid pour 150. Mostly the flame test detects. Flame tests and spectroscopy can be used to identify metal ions based on the unique colors they emit. How is it done?, under what. What metal ion causes fireworks to be red?
From ar.inspiredpencil.com
Flame Test Lab Answer Key Flame Test & Spectroscopy Answer Key To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and. Study with quizlet and memorize flashcards containing terms like what is a flame test? Observe the flame test for seven different metallic ions. The objectives of this lab are to: Mostly the. Flame Test & Spectroscopy Answer Key.
From studylib.net
FLAME TEST LAB PROCEDURE Flame Test & Spectroscopy Answer Key Flame tests and spectroscopy can be used to identify metal ions based on the unique colors they emit. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and. Study with quizlet and memorize flashcards containing terms like what is a flame test?. Flame Test & Spectroscopy Answer Key.
From studylib.net
6 Flame Test Lab Key Flame Test & Spectroscopy Answer Key Study with quizlet and memorize flashcards containing terms like what is a flame test? Use a flame test to determine which ion salt produces the red. 6m hydrochloric acid pour 150. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. Test the different metal salt solutions in a hot. Flame Test & Spectroscopy Answer Key.
From rocketsheets.co.uk
Flame Emission Spectroscopy Home Learning Worksheet GCSE rocketsheets Flame Test & Spectroscopy Answer Key Identify an unknown metallic element, using the flame test. Study with quizlet and memorize flashcards containing terms like what is a flame test? Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and. To perform flame tests of metal cations in order to observe their characteristic colors, to match the. Flame Test & Spectroscopy Answer Key.
From ar.inspiredpencil.com
Flame Test Lab Answer Key Flame Test & Spectroscopy Answer Key Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and. Flame tests and spectroscopy can be used to identify metal ions based on the unique colors they emit. When metal ion samples are heated in a flame test,. How is it done?, under what. Observe the flame test for seven. Flame Test & Spectroscopy Answer Key.
From ar.inspiredpencil.com
Flame Test Lab Answer Key Flame Test & Spectroscopy Answer Key Observe the flame test for seven different metallic ions. 6m hydrochloric acid pour 150. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and. Study with quizlet and memorize flashcards containing terms like what is a flame test? When metal ion samples are heated in a flame test,. The flame. Flame Test & Spectroscopy Answer Key.
From studylib.net
2nd 6wk Flame Test template Flame Test & Spectroscopy Answer Key Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and. Test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and to identify the. Observe the flame test for seven different metallic ions. How is it done?, under what.. Flame Test & Spectroscopy Answer Key.
From athensmutualaid.net
Flame Test Lab Answer Key Chemistry › Athens Mutual Student Corner Flame Test & Spectroscopy Answer Key Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and. The objectives of this lab are to: 6m hydrochloric acid pour 150. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and.. Flame Test & Spectroscopy Answer Key.
From www.animalia-life.club
Flame Test Lab Results Flame Test & Spectroscopy Answer Key The objectives of this lab are to: Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and. Observe the flame test for seven different metallic ions. Study with quizlet and memorize flashcards containing terms like what is a flame test? To perform flame tests of metal cations in order to. Flame Test & Spectroscopy Answer Key.
From www.chegg.com
Solved Flame Test & Spectroscopy Background Electrons are Flame Test & Spectroscopy Answer Key Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and. 6m hydrochloric acid pour 150. Mostly the flame test detects. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and. Use a. Flame Test & Spectroscopy Answer Key.
From athensmutualaid.net
Chemistry Flame Test Lab Answer Key Pdf › Athens Mutual Student Corner Flame Test & Spectroscopy Answer Key When metal ion samples are heated in a flame test,. Use a flame test to determine which ion salt produces the red. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. Flame tests and spectroscopy can be used to identify metal ions based on the unique colors they emit.. Flame Test & Spectroscopy Answer Key.
From www.chegg.com
Solved Flame Tests and Atomic Emission Spectroscopy PreLab Flame Test & Spectroscopy Answer Key Test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and to identify the. 6m hydrochloric acid pour 150. Use a flame test to determine which ion salt produces the red. Observe the flame test for seven different metallic ions. To perform flame tests of metal cations in order. Flame Test & Spectroscopy Answer Key.
From alison.perka.org
Flame Test And Emission Spectra Lab Answer Key alison Flame Test & Spectroscopy Answer Key How is it done?, under what. When metal ion samples are heated in a flame test,. 6m hydrochloric acid pour 150. Observe the flame test for seven different metallic ions. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and. Flame tests. Flame Test & Spectroscopy Answer Key.
From ar.inspiredpencil.com
Flame Test Lab Answer Key Flame Test & Spectroscopy Answer Key Use a flame test to determine which ion salt produces the red. How is it done?, under what. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and. Perform flame tests of metal cations in order to observe their characteristic colors, perform. Flame Test & Spectroscopy Answer Key.
From learningricardo.z19.web.core.windows.net
Flame Test Lab Worksheet Answer Key Chemistry Flame Test & Spectroscopy Answer Key Study with quizlet and memorize flashcards containing terms like what is a flame test? What metal ion causes fireworks to be red? Mostly the flame test detects. 6m hydrochloric acid pour 150. Identify an unknown metallic element, using the flame test. Use a flame test to determine which ion salt produces the red. To perform flame tests of metal cations. Flame Test & Spectroscopy Answer Key.
From myans.bhantedhammika.net
Flame Test Pre Lab Answer Key Flame Test & Spectroscopy Answer Key Flame tests and spectroscopy can be used to identify metal ions based on the unique colors they emit. Identify an unknown metallic element, using the flame test. Test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and to identify the. What metal ion causes fireworks to be red?. Flame Test & Spectroscopy Answer Key.
From www.chegg.com
Solved Flame Test & Spectroscopy Background Electrons are Flame Test & Spectroscopy Answer Key Mostly the flame test detects. Use a flame test to determine which ion salt produces the red. What metal ion causes fireworks to be red? When metal ion samples are heated in a flame test,. Identify an unknown metallic element, using the flame test. Flame tests and spectroscopy can be used to identify metal ions based on the unique colors. Flame Test & Spectroscopy Answer Key.
From www.scribd.com
Flame Test & Spectroscopy Virtual Lab PDF Emission Spectrum Flame Test & Spectroscopy Answer Key Use a flame test to determine which ion salt produces the red. Identify an unknown metallic element, using the flame test. Test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and to identify the. Flame tests and spectroscopy can be used to identify metal ions based on the. Flame Test & Spectroscopy Answer Key.
From studyzonecoxprospector.z14.web.core.windows.net
Chemistry Lab Flame Tests Activity Answer Key Flame Test & Spectroscopy Answer Key How is it done?, under what. Identify an unknown metallic element, using the flame test. Study with quizlet and memorize flashcards containing terms like what is a flame test? Flame tests and spectroscopy can be used to identify metal ions based on the unique colors they emit. Use a flame test to determine which ion salt produces the red. Perform. Flame Test & Spectroscopy Answer Key.
From lessonpage.z13.web.core.windows.net
Flame Test Lab Worksheet Flame Test & Spectroscopy Answer Key Identify an unknown metallic element, using the flame test. How is it done?, under what. Use a flame test to determine which ion salt produces the red. Flame tests and spectroscopy can be used to identify metal ions based on the unique colors they emit. When metal ion samples are heated in a flame test,. Mostly the flame test detects.. Flame Test & Spectroscopy Answer Key.
From athensmutualaid.net
Flame Test Lab Answer Key Pdf › Athens Mutual Student Corner Flame Test & Spectroscopy Answer Key Mostly the flame test detects. Observe the flame test for seven different metallic ions. Use a flame test to determine which ion salt produces the red. Test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and to identify the. The objectives of this lab are to: Identify an. Flame Test & Spectroscopy Answer Key.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Flame Test & Spectroscopy Answer Key Flame tests and spectroscopy can be used to identify metal ions based on the unique colors they emit. How is it done?, under what. What metal ion causes fireworks to be red? Identify an unknown metallic element, using the flame test. Test the different metal salt solutions in a hot flame and observe the characteristic color given off by each. Flame Test & Spectroscopy Answer Key.
From myans.bhantedhammika.net
Flame Test And Atomic Spectra Lab Answer Key Flame Test & Spectroscopy Answer Key To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and. Test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and to identify the. Observe the flame test for seven different. Flame Test & Spectroscopy Answer Key.
From myans.bhantedhammika.net
Atomic Emission And Flame Test Lab Answers Flame Test & Spectroscopy Answer Key Mostly the flame test detects. What metal ion causes fireworks to be red? How is it done?, under what. 6m hydrochloric acid pour 150. The objectives of this lab are to: Test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and to identify the. Identify an unknown metallic. Flame Test & Spectroscopy Answer Key.
From athensmutualaid.net
Flame Test And Atomic Spectra Lab Answer Key › Athens Mutual Student Corner Flame Test & Spectroscopy Answer Key How is it done?, under what. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. Study with quizlet and memorize flashcards containing terms like what is a flame test? When metal ion samples are heated in a flame test,. Use a flame test to determine which ion salt produces. Flame Test & Spectroscopy Answer Key.
From myans.bhantedhammika.net
Flame Test Pre Lab Answer Key Flame Test & Spectroscopy Answer Key Use a flame test to determine which ion salt produces the red. Flame tests and spectroscopy can be used to identify metal ions based on the unique colors they emit. Study with quizlet and memorize flashcards containing terms like what is a flame test? Test the different metal salt solutions in a hot flame and observe the characteristic color given. Flame Test & Spectroscopy Answer Key.
From education2research.com
The Ultimate Flame Tests Lab Answer Key Unlocking the Secrets of Flame Test & Spectroscopy Answer Key Identify an unknown metallic element, using the flame test. When metal ion samples are heated in a flame test,. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and. Use a flame test to determine which ion salt produces the red. To perform flame tests of metal cations in order. Flame Test & Spectroscopy Answer Key.
From www.chegg.com
Solved Flame Test & Spectroscopy Background Electrons are Flame Test & Spectroscopy Answer Key Test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and to identify the. Study with quizlet and memorize flashcards containing terms like what is a flame test? Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and. The. Flame Test & Spectroscopy Answer Key.
From www.chegg.com
Solved Flame Test & Spectroscopy Background Electrons are Flame Test & Spectroscopy Answer Key Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and. Flame tests and spectroscopy can be used to identify metal ions based on the unique colors they emit. Use a flame test to determine which ion salt produces the red. What metal ion causes fireworks to be red? Test the. Flame Test & Spectroscopy Answer Key.
From ar.inspiredpencil.com
Flame Test Lab Answer Key Flame Test & Spectroscopy Answer Key Mostly the flame test detects. When metal ion samples are heated in a flame test,. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. Study with quizlet and memorize flashcards containing terms like what is a flame test? What metal ion causes fireworks to be red? Observe the flame. Flame Test & Spectroscopy Answer Key.
From myans.bhantedhammika.net
Flame Test And Atomic Spectra Lab Answer Key Flame Test & Spectroscopy Answer Key Observe the flame test for seven different metallic ions. 6m hydrochloric acid pour 150. Test the different metal salt solutions in a hot flame and observe the characteristic color given off by each excited atom and to identify the. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission spectra. Perform. Flame Test & Spectroscopy Answer Key.
From knowledge.carolina.com
Flame Tests and Spectroscopy Get Excited About Color Carolina Flame Test & Spectroscopy Answer Key 6m hydrochloric acid pour 150. When metal ion samples are heated in a flame test,. What metal ion causes fireworks to be red? Flame tests and spectroscopy can be used to identify metal ions based on the unique colors they emit. The flame test is an analytical chemistry technique that helps identify elements in samples based on their characteristic emission. Flame Test & Spectroscopy Answer Key.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind Flame Test & Spectroscopy Answer Key 6m hydrochloric acid pour 150. Identify an unknown metallic element, using the flame test. Mostly the flame test detects. The objectives of this lab are to: What metal ion causes fireworks to be red? Observe the flame test for seven different metallic ions. Use a flame test to determine which ion salt produces the red. When metal ion samples are. Flame Test & Spectroscopy Answer Key.
From www.mrpalermo.com
Virtual Lab Flame Test & Spectroscopy Mr. Palermo's Flipped Flame Test & Spectroscopy Answer Key Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine the frequency and. The objectives of this lab are to: How is it done?, under what. 6m hydrochloric acid pour 150. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an. Flame Test & Spectroscopy Answer Key.
From www.scribd.com
Flame Test Lab Example Emission Spectrum Atoms Flame Test & Spectroscopy Answer Key What metal ion causes fireworks to be red? Observe the flame test for seven different metallic ions. To perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength of visible light, and. When metal ion samples are heated in a flame test,. The flame test is an. Flame Test & Spectroscopy Answer Key.