Magnesium Ion Chemical Charge . thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Mg 2+ cl − combining one ion of each does not. For example, iron( ii ) has a. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. the element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. The symbol for the ion is mg. roman numeral notation indicates charge of ion when element commonly forms more than one ion. Predict the charge of monatomic main group elements based on their group number. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:
from www.youtube.com
thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. the element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. Predict the charge of monatomic main group elements based on their group number. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. For example, iron( ii ) has a. The symbol for the ion is mg. Mg 2+ cl − combining one ion of each does not. roman numeral notation indicates charge of ion when element commonly forms more than one ion. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:
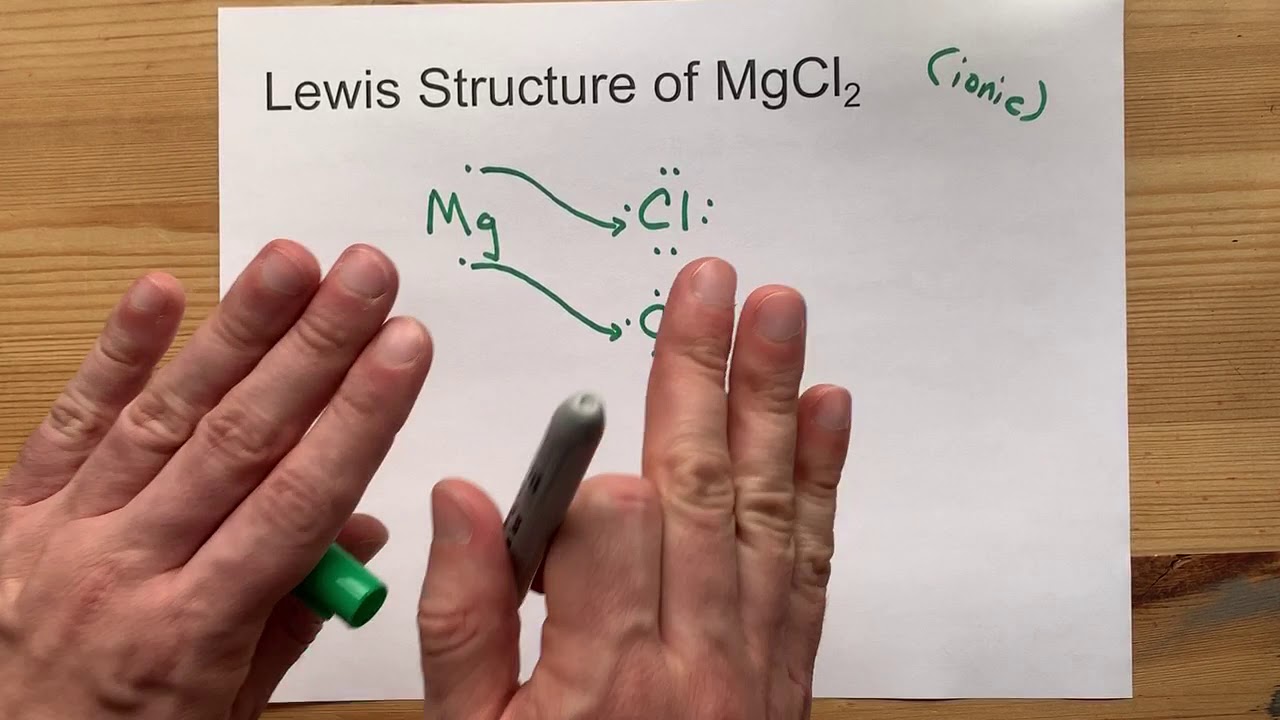
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube
Magnesium Ion Chemical Charge Mg 2+ cl − combining one ion of each does not. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: roman numeral notation indicates charge of ion when element commonly forms more than one ion. the element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. Mg 2+ cl − combining one ion of each does not. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. For example, iron( ii ) has a. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. Predict the charge of monatomic main group elements based on their group number. The symbol for the ion is mg. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:
From fr.freepik.com
Symbole de magnésium Élément chimique du tableau périodique Magnesium Ion Chemical Charge a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Mg 2+ cl − combining one ion of each does not. the element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. For example, iron( ii ) has a. . Magnesium Ion Chemical Charge.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Ion Chemical Charge thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. Predict the charge of monatomic main group elements based on their group number. For example,. Magnesium Ion Chemical Charge.
From www.researchgate.net
Schematic illustrations of the behavior of the magnesium ions at the Magnesium Ion Chemical Charge the element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. Mg 2+ cl − combining one ion of each does not. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: a magnesium ion has a 2+ charge,. Magnesium Ion Chemical Charge.
From www.thesciencehive.co.uk
The Periodic Table (GCSE) — the science hive Magnesium Ion Chemical Charge to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. Mg 2+ cl − combining one ion of each does not. Predict the charge of monatomic main group elements based on their group number. roman numeral notation indicates charge of ion. Magnesium Ion Chemical Charge.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Ion Chemical Charge \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. roman numeral. Magnesium Ion Chemical Charge.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Ion Chemical Charge Mg 2+ cl − combining one ion of each does not. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. roman numeral notation indicates charge of ion when element commonly forms more than one ion. a magnesium ion has. Magnesium Ion Chemical Charge.
From fyogfctqx.blob.core.windows.net
Magnesium The Charge at Katelyn Johnson blog Magnesium Ion Chemical Charge For example, iron( ii ) has a. Predict the charge of monatomic main group elements based on their group number. roman numeral notation indicates charge of ion when element commonly forms more than one ion. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: The symbol for the ion is mg. \[\ce{mg^{2+}cl^{−}}\]. Magnesium Ion Chemical Charge.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:1.6 4 Ionic Bonds Dot Magnesium Ion Chemical Charge thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is mg. the element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. to balance the charges with the lowest. Magnesium Ion Chemical Charge.
From www.alamy.com
Diagram to show ionic bonding in magnesium oxide Stock Vector Image Magnesium Ion Chemical Charge thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is mg. For example, iron( ii ) has a. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. . Magnesium Ion Chemical Charge.
From giohhzmmh.blob.core.windows.net
Magnesium Formula And Charge at Catherine Curran blog Magnesium Ion Chemical Charge a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: The symbol for the ion is mg. the element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. Predict the charge of monatomic main group elements based on their group. Magnesium Ion Chemical Charge.
From manuallistgranitise.z1.web.core.windows.net
Lewis Diagram Of Magnesium Magnesium Ion Chemical Charge The symbol for the ion is mg. roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron( ii ) has a. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. a magnesium ion has a 2+ charge, while a. Magnesium Ion Chemical Charge.
From www.youtube.com
Lewis Structure of Magnesium Oxide (MgO) YouTube Magnesium Ion Chemical Charge Predict the charge of monatomic main group elements based on their group number. The symbol for the ion is mg. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: roman numeral notation indicates charge of ion when element commonly forms more than one ion. thus, a magnesium atom will form a. Magnesium Ion Chemical Charge.
From schematicdatabitos99.z22.web.core.windows.net
Magnesium Atomic Structure Diagram Magnesium Ion Chemical Charge to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: the element magnesium is found in group two of the periodic table, so a magnesium. Magnesium Ion Chemical Charge.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Ion Chemical Charge a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: the element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: to balance. Magnesium Ion Chemical Charge.
From exycocyow.blob.core.windows.net
Magnesium V Ion at Jacqueline Sneed blog Magnesium Ion Chemical Charge The symbol for the ion is mg. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. roman numeral notation indicates charge of ion when element commonly forms more than one ion. the element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. a magnesium. Magnesium Ion Chemical Charge.
From giohhzmmh.blob.core.windows.net
Magnesium Formula And Charge at Catherine Curran blog Magnesium Ion Chemical Charge the element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. roman numeral notation indicates charge of ion when element commonly forms more than one ion. Predict the charge of monatomic main group elements based on their group number. to balance the charges with. Magnesium Ion Chemical Charge.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Magnesium Ion Chemical Charge to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. The symbol for the ion is mg. Mg 2+ cl − combining one ion of each does not. a magnesium ion has a 2+ charge, while a chlorine ion has a. Magnesium Ion Chemical Charge.
From giohhzmmh.blob.core.windows.net
Magnesium Formula And Charge at Catherine Curran blog Magnesium Ion Chemical Charge Mg 2+ cl − combining one ion of each does not. the element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. roman numeral notation indicates charge of ion when element commonly forms more than one ion. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not. Magnesium Ion Chemical Charge.
From gioogmqbg.blob.core.windows.net
Magnesium Charge Is at Albert Cooper blog Magnesium Ion Chemical Charge Mg 2+ cl − combining one ion of each does not. the element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not. Magnesium Ion Chemical Charge.
From www.youtube.com
Atomic Structure (Bohr Model) for Magnesium (Mg) YouTube Magnesium Ion Chemical Charge to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. The symbol for the ion is mg. Predict the charge of monatomic main group elements based on their group number. thus, a magnesium atom will form a cation with two fewer. Magnesium Ion Chemical Charge.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Magnesium Ion Chemical Charge a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: roman numeral notation indicates charge of ion when element commonly forms more than one ion. Predict the charge of monatomic main group elements based on their group number. a magnesium ion has a 2+ charge, while a chlorine ion has a 1−. Magnesium Ion Chemical Charge.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Magnesium Ion Chemical Charge thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. Mg 2+ cl − combining one ion of each does not. The symbol for the ion is mg. a magnesium ion has a 2+ charge, while a chlorine ion has. Magnesium Ion Chemical Charge.
From www.youtube.com
How to Find the Ionic Charge for Magnesium (Mg) YouTube Magnesium Ion Chemical Charge \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. Predict the charge of monatomic main group elements based on their group number. The symbol. Magnesium Ion Chemical Charge.
From giokrpnkk.blob.core.windows.net
Magnesium Nuclear Charge at Fabian Barter blog Magnesium Ion Chemical Charge Predict the charge of monatomic main group elements based on their group number. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: For example, iron( ii ) has a. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. to balance the. Magnesium Ion Chemical Charge.
From manuallistgranitise.z1.web.core.windows.net
Lewis Diagram For Magnesium Magnesium Ion Chemical Charge a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: The symbol for the ion is mg. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. Mg 2+ cl − combining one ion of each does not. the element magnesium is found in group two of the periodic table, so a magnesium ion. Magnesium Ion Chemical Charge.
From www.slideshare.net
Bonding Basics Magnesium Ion Chemical Charge The symbol for the ion is mg. Mg 2+ cl − combining one ion of each does not. the element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. Predict the charge of monatomic main group elements. Magnesium Ion Chemical Charge.
From www.myxxgirl.com
Atom Diagram Of Magnesium Diagram To Show Ionic Bonding In Magnesium Magnesium Ion Chemical Charge a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Mg 2+ cl − combining one ion of each does not. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. thus, a magnesium atom. Magnesium Ion Chemical Charge.
From www.newtondesk.com
Magnesium Mg (Elements 12) of Periodic Table Elements FlashCards Magnesium Ion Chemical Charge to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. For example, iron( ii ) has a. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. Magnesium Ion Chemical Charge.
From www.youtube.com
How to draw the Mg2+ Lewis Dot Structure. YouTube Magnesium Ion Chemical Charge \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Predict the charge of monatomic main group elements based on their group number. For example,. Magnesium Ion Chemical Charge.
From ar.inspiredpencil.com
Magnesium Ion Lewis Dot Structure Magnesium Ion Chemical Charge thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. roman numeral notation indicates charge of ion when element commonly forms more than one ion. The symbol for the ion is mg. the element magnesium is found in group. Magnesium Ion Chemical Charge.
From www.showme.com
Magnesium + Oxygen Ionic Bonding Science, Chemistry ShowMe Magnesium Ion Chemical Charge a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. thus, a magnesium atom will form. Magnesium Ion Chemical Charge.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Ion Chemical Charge the element magnesium is found in group two of the periodic table, so a magnesium ion is likely to have a two plus charge. \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. For example, iron( ii ) has a. Mg 2+ cl − combining one ion of each does not. to balance the charges with the lowest. Magnesium Ion Chemical Charge.
From galvinconanstuart.blogspot.com
Lewis Dot Diagram For Magnesium General Wiring Diagram Magnesium Ion Chemical Charge \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: Mg 2+ cl − combining one ion of each does not. to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on. Magnesium Ion Chemical Charge.
From brainly.in
EXPLAIN WHY MAGNESIUM FORMS MG ION Brainly.in Magnesium Ion Chemical Charge Mg 2+ cl − combining one ion of each does not. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: \[\ce{mg^{2+}cl^{−}}\] combining one ion of each does not completely. Predict the charge of monatomic main group elements based on their group number. to balance the charges with the lowest number of ions. Magnesium Ion Chemical Charge.
From www.youtube.com
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube Magnesium Ion Chemical Charge to balance the charges with the lowest number of ions possible, we need to have two chloride ions to balance the charge on the one magnesium ion. For example, iron( ii ) has a. a magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge: thus, a magnesium atom will form a cation. Magnesium Ion Chemical Charge.