Ar Definition Chemistry A Level . the mass spectrum and relative atomic mass. the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. The term relative formula mass will be. It can be used to find the. revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry experts at. Relative atomic mass (a r) is the weighted average mass. the relative atomic mass (ar) is the average mass of an atom of an element relative to one twelfth of the mass of an atom of. A mass spectrometer creates the mass spectrum. relative atomic mass (ar) is defined as: relative atomic masses (a r) are used to compare the masses of all elements on the periodic table. The mean mass of an atom of an element, divided by one twelfth of the mean mass. — relative atomic mass and relative molecular mass in terms of 12 c.
from www.linstitute.net
The term relative formula mass will be. It can be used to find the. The mean mass of an atom of an element, divided by one twelfth of the mean mass. the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. relative atomic masses (a r) are used to compare the masses of all elements on the periodic table. the relative atomic mass (ar) is the average mass of an atom of an element relative to one twelfth of the mass of an atom of. — relative atomic mass and relative molecular mass in terms of 12 c. relative atomic mass (ar) is defined as: revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry experts at. the mass spectrum and relative atomic mass.
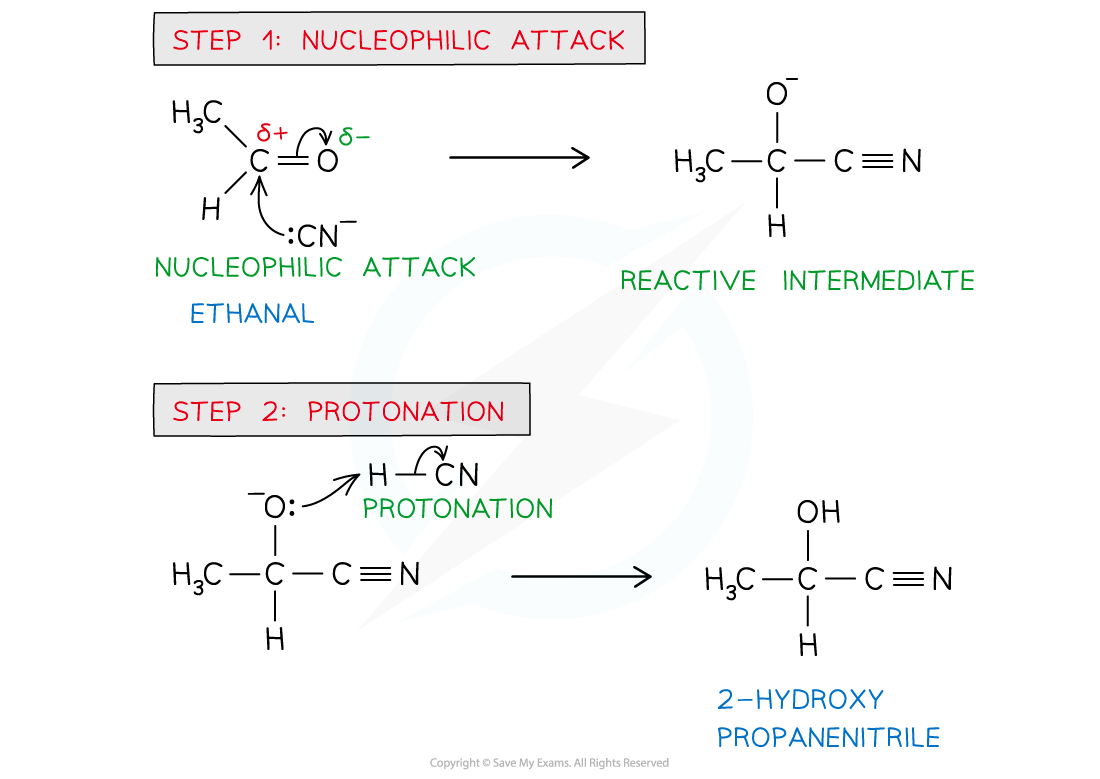
CIE A Level Chemistry复习笔记3.5.3 Reaction with HCN翰林国际教育
Ar Definition Chemistry A Level — relative atomic mass and relative molecular mass in terms of 12 c. the mass spectrum and relative atomic mass. The term relative formula mass will be. It can be used to find the. The mean mass of an atom of an element, divided by one twelfth of the mean mass. revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry experts at. A mass spectrometer creates the mass spectrum. relative atomic masses (a r) are used to compare the masses of all elements on the periodic table. Relative atomic mass (a r) is the weighted average mass. — relative atomic mass and relative molecular mass in terms of 12 c. the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. relative atomic mass (ar) is defined as: the relative atomic mass (ar) is the average mass of an atom of an element relative to one twelfth of the mass of an atom of.
From www.slideserve.com
PPT Relative atomic mass A r PowerPoint Presentation, free download Ar Definition Chemistry A Level the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. It can be used to find the. — relative atomic mass and relative molecular mass in terms of 12 c. The term relative formula mass will be. relative atomic mass (ar) is defined as: Relative atomic mass (a r) is the weighted. Ar Definition Chemistry A Level.
From www.youtube.com
applying AR in chemistry educationcase2 YouTube Ar Definition Chemistry A Level A mass spectrometer creates the mass spectrum. relative atomic masses (a r) are used to compare the masses of all elements on the periodic table. revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry experts at. the concept of relative masses in chemistry is. Ar Definition Chemistry A Level.
From www.slideshare.net
The Mole Ar And Mr Ar Definition Chemistry A Level revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry experts at. the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. The term relative formula mass will be. the mass spectrum and relative atomic mass. A mass spectrometer creates. Ar Definition Chemistry A Level.
From slideshare.net
The Mole Ar And Mr Ar Definition Chemistry A Level the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. It can be used to find the. The mean mass of an atom of an element, divided by one twelfth of the mean mass. revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written. Ar Definition Chemistry A Level.
From www.youtube.com
Chemistry AR developed by AR.TEAM YouTube Ar Definition Chemistry A Level A mass spectrometer creates the mass spectrum. relative atomic masses (a r) are used to compare the masses of all elements on the periodic table. the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. The term relative formula mass will be. relative atomic mass (ar) is defined as: — relative. Ar Definition Chemistry A Level.
From www.youtube.com
Chemistry AR guide YouTube Ar Definition Chemistry A Level A mass spectrometer creates the mass spectrum. relative atomic masses (a r) are used to compare the masses of all elements on the periodic table. revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry experts at. — relative atomic mass and relative molecular mass. Ar Definition Chemistry A Level.
From www.youtube.com
Atomic mass u, Da, Ar Solutions & Acidity meriSTEM YouTube Ar Definition Chemistry A Level revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry experts at. A mass spectrometer creates the mass spectrum. It can be used to find the. relative atomic mass (ar) is defined as: The mean mass of an atom of an element, divided by one twelfth. Ar Definition Chemistry A Level.
From www.linstitute.net
CIE A Level Chemistry复习笔记3.5.3 Reaction with HCN翰林国际教育 Ar Definition Chemistry A Level the relative atomic mass (ar) is the average mass of an atom of an element relative to one twelfth of the mass of an atom of. the mass spectrum and relative atomic mass. relative atomic mass (ar) is defined as: It can be used to find the. relative atomic masses (a r) are used to compare. Ar Definition Chemistry A Level.
From www.youtube.com
1.2 Define Relative Atomic Mass (Ar) and Relative Molecular Mass (Mr Ar Definition Chemistry A Level revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry experts at. the mass spectrum and relative atomic mass. The mean mass of an atom of an element, divided by one twelfth of the mean mass. relative atomic mass (ar) is defined as: the. Ar Definition Chemistry A Level.
From www.youtube.com
A Level Chemistry Revision "Relative Molecular Mass and Relative Ar Definition Chemistry A Level A mass spectrometer creates the mass spectrum. relative atomic mass (ar) is defined as: the relative atomic mass (ar) is the average mass of an atom of an element relative to one twelfth of the mass of an atom of. Relative atomic mass (a r) is the weighted average mass. relative atomic masses (a r) are used. Ar Definition Chemistry A Level.
From matthiashamann.work
AR Examples Chemistry Ar Definition Chemistry A Level relative atomic mass (ar) is defined as: — relative atomic mass and relative molecular mass in terms of 12 c. the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. The term relative formula mass will be. Relative atomic mass (a r) is the weighted average mass. the relative atomic mass. Ar Definition Chemistry A Level.
From www.compoundchem.com
A Brief Guide to Types of Isomerism in Organic Chemistry Compound Ar Definition Chemistry A Level It can be used to find the. revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry experts at. relative atomic mass (ar) is defined as: — relative atomic mass and relative molecular mass in terms of 12 c. The term relative formula mass will. Ar Definition Chemistry A Level.
From www.studocu.com
ALevel Chemistry Equation Sheet Equation Sheet for ALevel Chemistry Ar Definition Chemistry A Level the mass spectrum and relative atomic mass. A mass spectrometer creates the mass spectrum. It can be used to find the. the relative atomic mass (ar) is the average mass of an atom of an element relative to one twelfth of the mass of an atom of. The mean mass of an atom of an element, divided by. Ar Definition Chemistry A Level.
From devpost.com
AR Chemistry Learning Application Devpost Ar Definition Chemistry A Level relative atomic masses (a r) are used to compare the masses of all elements on the periodic table. the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. the relative atomic mass (ar) is the average mass of an atom of an element relative to one twelfth of the mass of an. Ar Definition Chemistry A Level.
From devpost.com
AR Chemistry Learning Application Devpost Ar Definition Chemistry A Level Relative atomic mass (a r) is the weighted average mass. A mass spectrometer creates the mass spectrum. relative atomic mass (ar) is defined as: — relative atomic mass and relative molecular mass in terms of 12 c. The term relative formula mass will be. the concept of relative masses in chemistry is essential for comprehending atomic and. Ar Definition Chemistry A Level.
From kotiwheel.weebly.com
Periodic table argon reactant definition chemistry kotiwheel Ar Definition Chemistry A Level relative atomic mass (ar) is defined as: It can be used to find the. Relative atomic mass (a r) is the weighted average mass. The term relative formula mass will be. The mean mass of an atom of an element, divided by one twelfth of the mean mass. revision notes on 1.2.1 relative atomic mass & relative molecular. Ar Definition Chemistry A Level.
From www.tes.com
Mass (Ar, Mr, Composition), GCSE Chemistry, Quantitative Chemistry Ar Definition Chemistry A Level relative atomic mass (ar) is defined as: the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. It can be used to find the. — relative atomic mass and relative molecular mass in terms of 12 c. The mean mass of an atom of an element, divided by one twelfth of the. Ar Definition Chemistry A Level.
From www.slideshare.net
The Mole Ar And Mr Ar Definition Chemistry A Level relative atomic masses (a r) are used to compare the masses of all elements on the periodic table. Relative atomic mass (a r) is the weighted average mass. revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry experts at. relative atomic mass (ar) is. Ar Definition Chemistry A Level.
From www.hanlin.com
CIE A Level Chemistry复习笔记4.1.3 Isotopic Abundance & Relative Atomic Ar Definition Chemistry A Level the mass spectrum and relative atomic mass. A mass spectrometer creates the mass spectrum. The term relative formula mass will be. Relative atomic mass (a r) is the weighted average mass. The mean mass of an atom of an element, divided by one twelfth of the mean mass. It can be used to find the. relative atomic masses. Ar Definition Chemistry A Level.
From www.tes.com
The Arrhenius Equation (A Level Chemistry) Teaching Resources Ar Definition Chemistry A Level the mass spectrum and relative atomic mass. the relative atomic mass (ar) is the average mass of an atom of an element relative to one twelfth of the mass of an atom of. relative atomic masses (a r) are used to compare the masses of all elements on the periodic table. The term relative formula mass will. Ar Definition Chemistry A Level.
From www.researchgate.net
A schematic diagram for energy level and transition of Ar atom. Argon Ar Definition Chemistry A Level revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry experts at. It can be used to find the. — relative atomic mass and relative molecular mass in terms of 12 c. the concept of relative masses in chemistry is essential for comprehending atomic and. Ar Definition Chemistry A Level.
From www.youtube.com
AR Chemistry Augmented Reality Education ( Tutorial ) Immersive Ar Definition Chemistry A Level revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry experts at. A mass spectrometer creates the mass spectrum. — relative atomic mass and relative molecular mass in terms of 12 c. Relative atomic mass (a r) is the weighted average mass. The mean mass of. Ar Definition Chemistry A Level.
From www.slideshare.net
The Mole Ar And Mr Ar Definition Chemistry A Level — relative atomic mass and relative molecular mass in terms of 12 c. revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry experts at. the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. The mean mass of an. Ar Definition Chemistry A Level.
From lukebissell.co.uk
AR Chemistry Ar Definition Chemistry A Level the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. the mass spectrum and relative atomic mass. the relative atomic mass (ar) is the average mass of an atom of an element relative to one twelfth of the mass of an atom of. revision notes on 1.2.1 relative atomic mass &. Ar Definition Chemistry A Level.
From www.youtube.com
AR Chemistry Augmented Reality Education YouTube Ar Definition Chemistry A Level — relative atomic mass and relative molecular mass in terms of 12 c. the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. relative atomic masses (a r) are used to compare the masses of all elements on the periodic table. the relative atomic mass (ar) is the average mass of. Ar Definition Chemistry A Level.
From thechemistrynotes.com
Argon (Ar) Element Properties, Amazing Uses, Facts Ar Definition Chemistry A Level — relative atomic mass and relative molecular mass in terms of 12 c. It can be used to find the. relative atomic mass (ar) is defined as: A mass spectrometer creates the mass spectrum. The term relative formula mass will be. revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level. Ar Definition Chemistry A Level.
From www.researchgate.net
Main energy levels for the Ar atom and the N2 molecule. Download Ar Definition Chemistry A Level relative atomic masses (a r) are used to compare the masses of all elements on the periodic table. Relative atomic mass (a r) is the weighted average mass. A mass spectrometer creates the mass spectrum. the mass spectrum and relative atomic mass. — relative atomic mass and relative molecular mass in terms of 12 c. the. Ar Definition Chemistry A Level.
From www.youtube.com
AR Chemistry YouTube Ar Definition Chemistry A Level The term relative formula mass will be. the mass spectrum and relative atomic mass. It can be used to find the. revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry experts at. relative atomic mass (ar) is defined as: The mean mass of an. Ar Definition Chemistry A Level.
From www.showme.com
Calculating Ar and Mr Science, Chemistry, Atoms ShowMe Ar Definition Chemistry A Level The mean mass of an atom of an element, divided by one twelfth of the mean mass. — relative atomic mass and relative molecular mass in terms of 12 c. Relative atomic mass (a r) is the weighted average mass. It can be used to find the. the mass spectrum and relative atomic mass. A mass spectrometer creates. Ar Definition Chemistry A Level.
From animalia-life.club
Gas Symbol Periodic Table Ar Definition Chemistry A Level It can be used to find the. the mass spectrum and relative atomic mass. The term relative formula mass will be. relative atomic mass (ar) is defined as: the relative atomic mass (ar) is the average mass of an atom of an element relative to one twelfth of the mass of an atom of. relative atomic. Ar Definition Chemistry A Level.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记1.6.5 Amount of Substance Calculations翰林国际教育 Ar Definition Chemistry A Level Relative atomic mass (a r) is the weighted average mass. the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. The mean mass of an atom of an element, divided by one twelfth of the mean mass. revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level. Ar Definition Chemistry A Level.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.19 carry out mole calculations using Ar Definition Chemistry A Level the mass spectrum and relative atomic mass. The term relative formula mass will be. the relative atomic mass (ar) is the average mass of an atom of an element relative to one twelfth of the mass of an atom of. — relative atomic mass and relative molecular mass in terms of 12 c. the concept of. Ar Definition Chemistry A Level.
From www.researchgate.net
4. Argon energy level diagram and lowerlying effective levels Ar Definition Chemistry A Level The term relative formula mass will be. the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. relative atomic mass (ar) is defined as: It can be used to find the. Relative atomic mass (a r) is the weighted average mass. The mean mass of an atom of an element, divided by one. Ar Definition Chemistry A Level.
From www.youtube.com
AR Chemistry YouTube Ar Definition Chemistry A Level The term relative formula mass will be. Relative atomic mass (a r) is the weighted average mass. The mean mass of an atom of an element, divided by one twelfth of the mean mass. A mass spectrometer creates the mass spectrum. revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus,. Ar Definition Chemistry A Level.
From www.slideserve.com
PPT Chapter 15 Benzene and Aromaticity PowerPoint Presentation, free Ar Definition Chemistry A Level — relative atomic mass and relative molecular mass in terms of 12 c. It can be used to find the. the concept of relative masses in chemistry is essential for comprehending atomic and molecular structures. revision notes on 1.2.1 relative atomic mass & relative molecular mass for the aqa a level chemistry syllabus, written by the chemistry. Ar Definition Chemistry A Level.