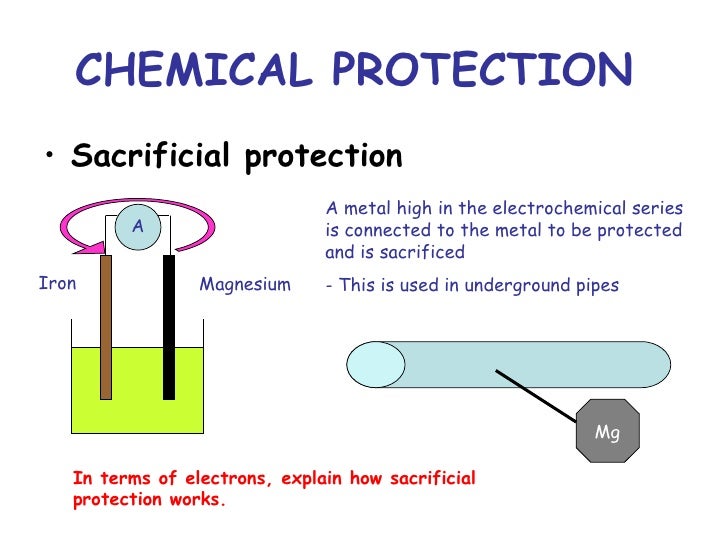
How Does Sacrificial Protection Prevent Rusting . Zinc is more reactive than iron, so it also acts as a sacrificial metal. Cathodic protection is a method that borrows from the principle of a battery, employing an alternative metal to serve as a sacrificial anode in place of iron. They are more reactive than iron and lose. A reactivity series lists metals in order of how reactive they are. The deterioration of metals through oxidation is a galvanic process called corrosion. Magnesium and zinc are often used as. Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. Corrosion is a galvanic process that can be prevented using cathodic protection. Sacrificial anodes are highly active metals that are used to prevent a less active material surface from corroding. Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. A sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from corrosion or. The zinc layer stops oxygen and water reaching the iron.
from www.slideshare.net
Corrosion is a galvanic process that can be prevented using cathodic protection. A reactivity series lists metals in order of how reactive they are. Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. They are more reactive than iron and lose. Zinc is more reactive than iron, so it also acts as a sacrificial metal. The deterioration of metals through oxidation is a galvanic process called corrosion. Magnesium and zinc are often used as. Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. The zinc layer stops oxygen and water reaching the iron. Sacrificial anodes are highly active metals that are used to prevent a less active material surface from corroding.
Corrosion, standard grade chemistry
How Does Sacrificial Protection Prevent Rusting The zinc layer stops oxygen and water reaching the iron. Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. Zinc is more reactive than iron, so it also acts as a sacrificial metal. Corrosion is a galvanic process that can be prevented using cathodic protection. A sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from corrosion or. A reactivity series lists metals in order of how reactive they are. They are more reactive than iron and lose. Sacrificial anodes are highly active metals that are used to prevent a less active material surface from corroding. The zinc layer stops oxygen and water reaching the iron. The deterioration of metals through oxidation is a galvanic process called corrosion. Magnesium and zinc are often used as. Cathodic protection is a method that borrows from the principle of a battery, employing an alternative metal to serve as a sacrificial anode in place of iron.
From www.youtube.com
How to Prevent Rusting? II/Preventing Rusting II/Galvanization Process How Does Sacrificial Protection Prevent Rusting A sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from corrosion or. Zinc is more reactive than iron, so it also acts as a sacrificial metal. Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used. How Does Sacrificial Protection Prevent Rusting.
From www.jetlube.com
5 Rust Prevention Tips for Your Metal Parts, Machines and Equipment How Does Sacrificial Protection Prevent Rusting A reactivity series lists metals in order of how reactive they are. The zinc layer stops oxygen and water reaching the iron. Corrosion is a galvanic process that can be prevented using cathodic protection. A sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from corrosion or. Zinc is. How Does Sacrificial Protection Prevent Rusting.
From www.nagwa.com
Question Video Identifying the Prevention of Rusting by a Current from How Does Sacrificial Protection Prevent Rusting Zinc is more reactive than iron, so it also acts as a sacrificial metal. The deterioration of metals through oxidation is a galvanic process called corrosion. A reactivity series lists metals in order of how reactive they are. Cathodic protection is a method that borrows from the principle of a battery, employing an alternative metal to serve as a sacrificial. How Does Sacrificial Protection Prevent Rusting.
From wd40.africa
Five Reasons Why Rust Treatment Is So Important WD40 Africa How Does Sacrificial Protection Prevent Rusting Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. They are more reactive than iron and lose. The zinc layer stops oxygen and water reaching the iron. Corrosion is a galvanic process that can be prevented using cathodic protection. A reactivity series lists metals in order of how reactive they are. The deterioration. How Does Sacrificial Protection Prevent Rusting.
From ar.inspiredpencil.com
Methods Of Prevention Of Rusting Of Iron How Does Sacrificial Protection Prevent Rusting Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. A reactivity series lists metals in order of how reactive they are. Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. Corrosion is a galvanic process that can be. How Does Sacrificial Protection Prevent Rusting.
From www.youtube.com
Get Rid of Rust DIY Rust Removal Using Metal Rescue YouTube How Does Sacrificial Protection Prevent Rusting Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. Zinc is more reactive than iron, so it also acts as a sacrificial metal. Sacrificial anodes are highly active metals that are used to prevent a less active material surface from corroding. A reactivity series lists metals in order of how reactive they are.. How Does Sacrificial Protection Prevent Rusting.
From galvanizing.org.uk
Barrier and sacrificial protection How Does Sacrificial Protection Prevent Rusting Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. The zinc layer stops oxygen and water reaching the iron. The deterioration of metals through oxidation is a galvanic process called corrosion. Zinc is more reactive than iron, so it also acts as a sacrificial metal. A reactivity series lists metals in order of. How Does Sacrificial Protection Prevent Rusting.
From byjus.com
Corrosion Rusting Of Iron, Rust Prevention & Preventive measures How Does Sacrificial Protection Prevent Rusting Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. A reactivity series lists metals in order of how reactive they are. The deterioration of metals through oxidation is a galvanic process called corrosion. Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will. How Does Sacrificial Protection Prevent Rusting.
From www.slideserve.com
PPT Corrosion, Rusting and How to Fight it. PowerPoint Presentation How Does Sacrificial Protection Prevent Rusting A sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from corrosion or. Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. A reactivity series lists metals in order of how reactive they are.. How Does Sacrificial Protection Prevent Rusting.
From metalscut4u.com
4 Easiest Way to Remove and Prevent Rust on Metal How Does Sacrificial Protection Prevent Rusting Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. They are more reactive than iron and lose. A reactivity series lists metals in order of how reactive they are. Corrosion is a galvanic process that can be prevented using cathodic protection. Magnesium and zinc are. How Does Sacrificial Protection Prevent Rusting.
From studylib.net
How to Prevent White Rust on Zinc Coated Steel How Does Sacrificial Protection Prevent Rusting Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. Magnesium and zinc are often used as. A reactivity series lists metals in order of how reactive they are. Cathodic protection is a method that borrows from the principle of a battery, employing an alternative metal to serve as a sacrificial anode in place. How Does Sacrificial Protection Prevent Rusting.
From www.tdk.com
How We Prevent Rust with Cathodic Protection and Sacrificial Anodes|The How Does Sacrificial Protection Prevent Rusting A sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from corrosion or. Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. Zinc is more reactive than iron, so it also acts as a sacrificial metal. Cathodic protection is a method that. How Does Sacrificial Protection Prevent Rusting.
From www.slideserve.com
PPT Experiment on HOW RUSTING CAN BE PREVENTED BY USING SACRIFICIAL How Does Sacrificial Protection Prevent Rusting Sacrificial anodes are highly active metals that are used to prevent a less active material surface from corroding. A sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from corrosion or. Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. The deterioration. How Does Sacrificial Protection Prevent Rusting.
From www.dixonpilot.com
Most Effective Ways To Prevent Rust The Dixon Pilot How Does Sacrificial Protection Prevent Rusting Zinc is more reactive than iron, so it also acts as a sacrificial metal. Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. The zinc layer stops oxygen and water reaching the iron. They are more reactive than iron and lose. Magnesium and zinc are often used as. A sacrificial metal is a. How Does Sacrificial Protection Prevent Rusting.
From www.researchgate.net
Schematic showing cathodic protection methods using sacrificial anode How Does Sacrificial Protection Prevent Rusting Magnesium and zinc are often used as. Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. They are more reactive than iron and lose. Sacrificial anodes are highly active. How Does Sacrificial Protection Prevent Rusting.
From glossary.periodni.com
Sacrificial protection Chemistry Dictionary & Glossary How Does Sacrificial Protection Prevent Rusting A reactivity series lists metals in order of how reactive they are. Zinc is more reactive than iron, so it also acts as a sacrificial metal. Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. The zinc layer stops oxygen and water reaching the iron. Sacrificial anodes are created from a metal alloy. How Does Sacrificial Protection Prevent Rusting.
From www.slideserve.com
PPT Extraction of metals PowerPoint Presentation ID208800 How Does Sacrificial Protection Prevent Rusting Sacrificial anodes are highly active metals that are used to prevent a less active material surface from corroding. Zinc is more reactive than iron, so it also acts as a sacrificial metal. They are more reactive than iron and lose. The zinc layer stops oxygen and water reaching the iron. Corrosion is a galvanic process that can be prevented using. How Does Sacrificial Protection Prevent Rusting.
From www.youtube.com
How To Remove Rust Treating & Preventing Rust on R&D Corner from How Does Sacrificial Protection Prevent Rusting Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. A reactivity series lists metals in order of how reactive they are. A sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from corrosion or. Cathodic protection is a method that borrows from. How Does Sacrificial Protection Prevent Rusting.
From www.youtube.com
STOP RUST! Rust Prevention in the YouTube How Does Sacrificial Protection Prevent Rusting The deterioration of metals through oxidation is a galvanic process called corrosion. A sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from corrosion or. Cathodic protection is a method that borrows from the principle of a battery, employing an alternative metal to serve as a sacrificial anode in. How Does Sacrificial Protection Prevent Rusting.
From www.autolovins.com
How to Stop Rust on your Car? Expert Guideline to Stop Rust How Does Sacrificial Protection Prevent Rusting Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. The zinc layer stops oxygen and water reaching the iron. The deterioration of metals through oxidation is a galvanic process called corrosion. Cathodic protection is a method that borrows from the principle of a battery, employing. How Does Sacrificial Protection Prevent Rusting.
From www.youtube.com
Impressed current Cathodic Protection How these metals sacrifice How Does Sacrificial Protection Prevent Rusting A sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from corrosion or. They are more reactive than iron and lose. Corrosion is a galvanic process that can be prevented using cathodic protection. Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the. How Does Sacrificial Protection Prevent Rusting.
From resin-expert.com
How to Stop Rust A Comprehensive Guide on Rust Prevention How Does Sacrificial Protection Prevent Rusting Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. Zinc is more reactive than iron, so it also acts as a sacrificial metal. The deterioration of metals through oxidation is a galvanic process called corrosion. Cathodic protection is a method that borrows from the principle of a battery, employing an alternative metal to. How Does Sacrificial Protection Prevent Rusting.
From www.flexp.com
Corrosion Prevention Corrosion Management Safety Equipment FlexPAC How Does Sacrificial Protection Prevent Rusting Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. The zinc layer stops oxygen and water reaching the iron. A reactivity series lists metals in order of how reactive they are. Corrosion is a galvanic process that can be prevented using cathodic protection. Cathodic protection. How Does Sacrificial Protection Prevent Rusting.
From www.slideserve.com
PPT Corrosion, Rusting and How to Fight it. PowerPoint Presentation How Does Sacrificial Protection Prevent Rusting A sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from corrosion or. The zinc layer stops oxygen and water reaching the iron. Cathodic protection is a method that borrows from the principle of a battery, employing an alternative metal to serve as a sacrificial anode in place of. How Does Sacrificial Protection Prevent Rusting.
From www.slideserve.com
PPT Experiment on HOW RUSTING CAN BE PREVENTED BY USING SACRIFICIAL How Does Sacrificial Protection Prevent Rusting Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. Magnesium and zinc are often used as. They are more reactive than iron and lose. The deterioration of metals through oxidation is a galvanic process called corrosion. A sacrificial metal is a metal used as a. How Does Sacrificial Protection Prevent Rusting.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 2.19 Understand How the Rusting of Iron May be How Does Sacrificial Protection Prevent Rusting Magnesium and zinc are often used as. Cathodic protection is a method that borrows from the principle of a battery, employing an alternative metal to serve as a sacrificial anode in place of iron. Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. Corrosion is a galvanic process that can be prevented using. How Does Sacrificial Protection Prevent Rusting.
From www.slideserve.com
PPT Extraction of metals PowerPoint Presentation, free download ID How Does Sacrificial Protection Prevent Rusting Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. The zinc layer stops oxygen and water reaching the iron. Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. The deterioration of metals through oxidation is a galvanic process. How Does Sacrificial Protection Prevent Rusting.
From www.slideshare.net
Corrosion, standard grade chemistry How Does Sacrificial Protection Prevent Rusting Cathodic protection is a method that borrows from the principle of a battery, employing an alternative metal to serve as a sacrificial anode in place of iron. The zinc layer stops oxygen and water reaching the iron. The deterioration of metals through oxidation is a galvanic process called corrosion. A sacrificial metal is a metal used as a sacrificial anode. How Does Sacrificial Protection Prevent Rusting.
From fyodagvzo.blob.core.windows.net
How Does Oiling And Greasing Prevent Rusting at Charles Randel blog How Does Sacrificial Protection Prevent Rusting Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. Cathodic protection is a method that borrows from the principle of a battery, employing an alternative metal to serve as a sacrificial anode in place of iron. Zinc is more reactive than iron, so it also. How Does Sacrificial Protection Prevent Rusting.
From www.toppr.com
Zn acts as sacrificial or Cathodic protect ion to prevent rusting of How Does Sacrificial Protection Prevent Rusting A reactivity series lists metals in order of how reactive they are. Corrosion is a galvanic process that can be prevented using cathodic protection. Zinc is more reactive than iron, so it also acts as a sacrificial metal. Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. Magnesium and zinc are often used. How Does Sacrificial Protection Prevent Rusting.
From www.slideserve.com
PPT Revision on soap and detergent PowerPoint Presentation, free How Does Sacrificial Protection Prevent Rusting A reactivity series lists metals in order of how reactive they are. Corrosion is a galvanic process that can be prevented using cathodic protection. A sacrificial metal is a metal used as a sacrificial anode in cathodic protection that corrodes to prevent a primary metal from corrosion or. Magnesium and zinc are often used as. The deterioration of metals through. How Does Sacrificial Protection Prevent Rusting.
From maritimepage.com
Sacrificial Anodes A Guide To Marine Corrosion Protection How Does Sacrificial Protection Prevent Rusting The deterioration of metals through oxidation is a galvanic process called corrosion. Magnesium and zinc are often used as. Corrosion is a galvanic process that can be prevented using cathodic protection. They are more reactive than iron and lose. Sacrificial anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be. How Does Sacrificial Protection Prevent Rusting.
From www.slideserve.com
PPT Experiment on HOW RUSTING CAN BE PREVENTED BY USING SACRIFICIAL How Does Sacrificial Protection Prevent Rusting Sacrificial anodes are highly active metals that are used to prevent a less active material surface from corroding. The zinc layer stops oxygen and water reaching the iron. Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. A reactivity series lists metals in order of how reactive they are. Cathodic protection is a. How Does Sacrificial Protection Prevent Rusting.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation, free How Does Sacrificial Protection Prevent Rusting Magnesium and zinc are often used as. Sacrificial protection is a corrosion protection method in which a more electrochemically active metal is electrically. The deterioration of metals through oxidation is a galvanic process called corrosion. Cathodic protection is a method that borrows from the principle of a battery, employing an alternative metal to serve as a sacrificial anode in place. How Does Sacrificial Protection Prevent Rusting.
From www.researchgate.net
7) Sacrificial Anode Cathodic Protection (SACP) Download Scientific How Does Sacrificial Protection Prevent Rusting They are more reactive than iron and lose. A reactivity series lists metals in order of how reactive they are. Sacrificial anodes are highly active metals that are used to prevent a less active material surface from corroding. Magnesium and zinc are often used as. Corrosion is a galvanic process that can be prevented using cathodic protection. The deterioration of. How Does Sacrificial Protection Prevent Rusting.