Magnesium Bicarbonate Anion . Diagnostic approach to metabolic ph abnormalities. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. All the anions are of this type, gaining the number of electrons. $\begingroup$ typically dissociation refers to the ions that are formed when a substance. June 29, 2024 by josh farkas.
from www.chegg.com
Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. $\begingroup$ typically dissociation refers to the ions that are formed when a substance. All the anions are of this type, gaining the number of electrons. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. June 29, 2024 by josh farkas. Diagnostic approach to metabolic ph abnormalities. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation.
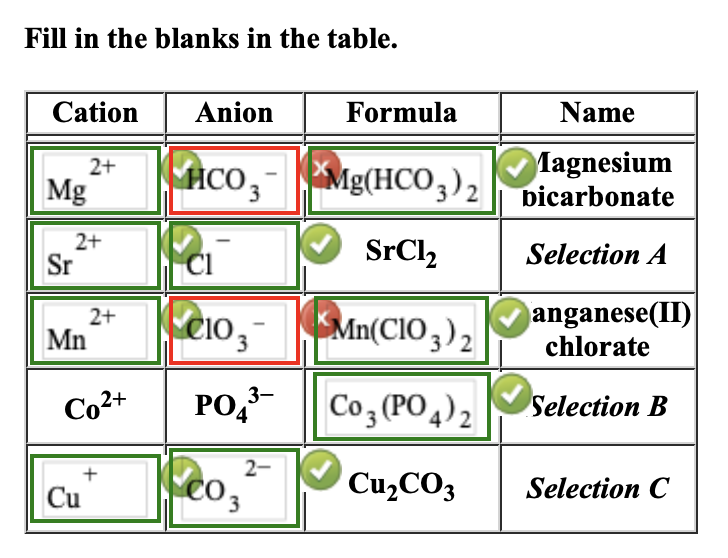
Solved Fill in the blanks in the table. Cation Anion Formula
Magnesium Bicarbonate Anion All the anions are of this type, gaining the number of electrons. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. $\begingroup$ typically dissociation refers to the ions that are formed when a substance. June 29, 2024 by josh farkas. All the anions are of this type, gaining the number of electrons. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. Diagnostic approach to metabolic ph abnormalities. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation.
From robot.ekstrabladet.dk
Cátions E ânions Tabela Magnesium Bicarbonate Anion Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. All. Magnesium Bicarbonate Anion.
From www.youtube.com
How to write the formula for magnesium bicarbonate YouTube Magnesium Bicarbonate Anion Diagnostic approach to metabolic ph abnormalities. Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. June 29,. Magnesium Bicarbonate Anion.
From edurev.in
A) Show the formation of magnesium oxide by the transfer of electrons B Magnesium Bicarbonate Anion June 29, 2024 by josh farkas. All the anions are of this type, gaining the number of electrons. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. $\begingroup$ typically dissociation refers to the ions. Magnesium Bicarbonate Anion.
From mavink.com
Common Anions And Cations Table Magnesium Bicarbonate Anion $\begingroup$ typically dissociation refers to the ions that are formed when a substance. Diagnostic approach to metabolic ph abnormalities. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. Figure 2.7.2 lists the ions (cation. Magnesium Bicarbonate Anion.
From www.chegg.com
Solved Fill in the blanks in the table. Cation Anion Formula Magnesium Bicarbonate Anion $\begingroup$ typically dissociation refers to the ions that are formed when a substance. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. All the anions are of this type, gaining the number of electrons. Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. Magnesium bicarbonate can be formed when magnesium. Magnesium Bicarbonate Anion.
From www.biocoherence.eu
Magnesium Bicarbonate Biocoherence Nederland Magnesium Bicarbonate Anion All the anions are of this type, gaining the number of electrons. Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. $\begingroup$ typically dissociation refers to the ions that are formed when a substance. Diagnostic approach to metabolic ph abnormalities. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Magnesium. Magnesium Bicarbonate Anion.
From www.dreamstime.com
Bicarbonate Anion, Chemical Structure. Common Salts Include Sodium Magnesium Bicarbonate Anion Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. Diagnostic approach to metabolic ph abnormalities. June 29, 2024 by josh farkas. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation. Magnesium Bicarbonate Anion.
From cartoondealer.com
Bicarbonate Anion Molecule Skeletal Formula, Chemical Structure. Vector Magnesium Bicarbonate Anion All the anions are of this type, gaining the number of electrons. June 29, 2024 by josh farkas. Diagnostic approach to metabolic ph abnormalities. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. $\begingroup$ typically dissociation refers to the ions that are formed when a substance. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen. Magnesium Bicarbonate Anion.
From www.vectorstock.com
Bicarbonate anion molecule chemical formula Vector Image Magnesium Bicarbonate Anion June 29, 2024 by josh farkas. Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. $\begingroup$ typically dissociation refers to the ions that are formed when a substance. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo). Magnesium Bicarbonate Anion.
From ponorevival.com
Pono Revival Magnesium Bicarbonate Water Electrolyte Balance Magnesium Bicarbonate Anion Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. June 29, 2024 by josh farkas. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. All the anions are of this. Magnesium Bicarbonate Anion.
From www.dreamstime.com
Bicarbonate Anion, Chemical Structure. Common Salts Include Sodium Magnesium Bicarbonate Anion All the anions are of this type, gaining the number of electrons. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula. Magnesium Bicarbonate Anion.
From cartoondealer.com
Bicarbonate Anion Molecule Skeletal Formula, Chemical Structure. Vector Magnesium Bicarbonate Anion Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide. Magnesium Bicarbonate Anion.
From www.biocoherence.eu
Magnesium bicarbonate Biocoherence Nederland Magnesium Bicarbonate Anion Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. Diagnostic approach to metabolic ph abnormalities. June 29, 2024 by josh farkas. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. All the anions are of this type, gaining the number of. Magnesium Bicarbonate Anion.
From www.numerade.com
SOLVEDFill out the missing information. Names must use proper IUPAC Magnesium Bicarbonate Anion Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. June 29, 2024 by josh farkas. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. $\begingroup$ typically dissociation refers to the ions that are formed when a substance. Diagnostic approach. Magnesium Bicarbonate Anion.
From www.vedantu.com
Magnesium Bicarbonate Learn Important Terms and Concepts Magnesium Bicarbonate Anion June 29, 2024 by josh farkas. Diagnostic approach to metabolic ph abnormalities. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. All the anions are of this type, gaining the number of. Magnesium Bicarbonate Anion.
From www.youtube.com
Magnesium Bicarbonate Water (How I Make It) YouTube Magnesium Bicarbonate Anion Diagnostic approach to metabolic ph abnormalities. $\begingroup$ typically dissociation refers to the ions that are formed when a substance. All the anions are of this type, gaining the number of electrons. Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Magnesium. Magnesium Bicarbonate Anion.
From www.alamy.com
Bicarbonate anion, chemical structure. Common salts include sodium Magnesium Bicarbonate Anion All the anions are of this type, gaining the number of electrons. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states.. Magnesium Bicarbonate Anion.
From giogwdfqm.blob.core.windows.net
Magnesium Bicarbonate Cation And Anion Formula at Heather Strickland blog Magnesium Bicarbonate Anion $\begingroup$ typically dissociation refers to the ions that are formed when a substance. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. June 29, 2024 by josh farkas. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. Magnesium bicarbonate or magnesium. Magnesium Bicarbonate Anion.
From pixels.com
Bicarbonate Anion Chemical Structure Photograph by Molekuul/science Magnesium Bicarbonate Anion Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. June 29, 2024 by josh farkas. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2. Magnesium Bicarbonate Anion.
From www.dreamstime.com
Bicarbonate Molecule 3d, Molecular Structure, Ball and Stick Model Magnesium Bicarbonate Anion All the anions are of this type, gaining the number of electrons. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. Diagnostic approach to metabolic ph abnormalities. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and. Magnesium Bicarbonate Anion.
From pixels.com
Bicarbonate Anion Chemical Structure Photograph by Molekuul/science Magnesium Bicarbonate Anion Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. Diagnostic approach to metabolic ph abnormalities. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing. Magnesium Bicarbonate Anion.
From cartoondealer.com
Bicarbonate, Molecular Structures, Polyatomic Anion, 3d Model Magnesium Bicarbonate Anion Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. Figure. Magnesium Bicarbonate Anion.
From www.toppr.com
14. A salt of sodium on reacting with magnesium chloride gives white Magnesium Bicarbonate Anion Diagnostic approach to metabolic ph abnormalities. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. All the anions are of this type, gaining the number of electrons. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. Magnesium bicarbonate, also known by. Magnesium Bicarbonate Anion.
From www.numerade.com
SOLVED Text Complete the table Anion symbol Formula Cation symbol Magnesium Bicarbonate Anion Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. June 29, 2024 by josh farkas. All the anions are of this type, gaining the number of electrons. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. $\begingroup$ typically dissociation refers to the ions that are formed when a substance. Magnesium. Magnesium Bicarbonate Anion.
From www.vectorstock.com
Bicarbonate anion chemical structure common salts Vector Image Magnesium Bicarbonate Anion All the anions are of this type, gaining the number of electrons. Diagnostic approach to metabolic ph abnormalities. $\begingroup$ typically dissociation refers to the ions that are formed when a substance. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo. Magnesium Bicarbonate Anion.
From www.numerade.com
SOLVED Cation Anion Formula Name Mg2+ Bicarbonate Mg(HCO3)2 Magnesium Magnesium Bicarbonate Anion $\begingroup$ typically dissociation refers to the ions that are formed when a substance. Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. Figure 2.7.2 lists the ions (cation and anion). Magnesium Bicarbonate Anion.
From www.dreamstime.com
Bicarbonate Anion Molecule Skeletal Formula, Chemical Structure. Stock Magnesium Bicarbonate Anion $\begingroup$ typically dissociation refers to the ions that are formed when a substance. Diagnostic approach to metabolic ph abnormalities. Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. All the. Magnesium Bicarbonate Anion.
From pristinehydro.com
Magnesium Bicarbonate a Paradigm Shift PristineHydro® Magnesium Bicarbonate Anion Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. June 29, 2024 by josh farkas. All the anions are of this type, gaining the number of electrons. Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co. Magnesium Bicarbonate Anion.
From www.numerade.com
2.117 Fill in the blanks in the table. Cation Anion Formula Name coro Magnesium Bicarbonate Anion Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Diagnostic approach to metabolic ph abnormalities. June 29, 2024 by josh farkas. All the anions are of this type, gaining the number of electrons. $\begingroup$ typically dissociation refers to the ions that are formed when a substance. Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is. Magnesium Bicarbonate Anion.
From www.dreamstime.com
Magnesium Carbonate Molecule Stock Vector Illustration of Magnesium Bicarbonate Anion Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts with carbon dioxide (co2) in water, leading to the formation. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. Figure. Magnesium Bicarbonate Anion.
From www.chegg.com
Solved Fill in the blanks in the table. Cation Anion Formula Magnesium Bicarbonate Anion All the anions are of this type, gaining the number of electrons. Diagnostic approach to metabolic ph abnormalities. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. Figure 2.7.2 lists the ions (cation and. Magnesium Bicarbonate Anion.
From healthjade.com
Anion gap calculation, anion gap blood test & causes of high or low Magnesium Bicarbonate Anion $\begingroup$ typically dissociation refers to the ions that are formed when a substance. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Diagnostic approach to metabolic ph abnormalities. All the anions are of this type, gaining the number of electrons. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate. Magnesium Bicarbonate Anion.
From www.youtube.com
How to Write the Formula for Magnesium bicarbonate (Magnesium hydrogen Magnesium Bicarbonate Anion Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. June 29, 2024 by josh farkas. Diagnostic approach to metabolic ph abnormalities. Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3). Magnesium Bicarbonate Anion.
From www.chemistrylearner.com
Magnesium Bicarbonate Facts, Formula, Synthesis, Properties, Uses Magnesium Bicarbonate Anion Magnesium bicarbonate, also known by its iupac name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula c 2 h 2 mgo 6 or mg(hco 3) 2 [1]. All the anions are of this type, gaining the number of electrons. Diagnostic approach to metabolic ph abnormalities. Figure 2.7.2 lists the ions (cation and. Magnesium Bicarbonate Anion.
From www.numerade.com
SOLVEDFill the blanks in the following table. Cation Anion Formula Magnesium Bicarbonate Anion Magnesium bicarbonate or magnesium hydrogencarbonate, mg(h co 3) 2, is the bicarbonate salt of magnesium. $\begingroup$ typically dissociation refers to the ions that are formed when a substance. June 29, 2024 by josh farkas. All the anions are of this type, gaining the number of electrons. Magnesium bicarbonate can be formed when magnesium hydroxide (mg(oh)2) or magnesium oxide (mgo) reacts. Magnesium Bicarbonate Anion.