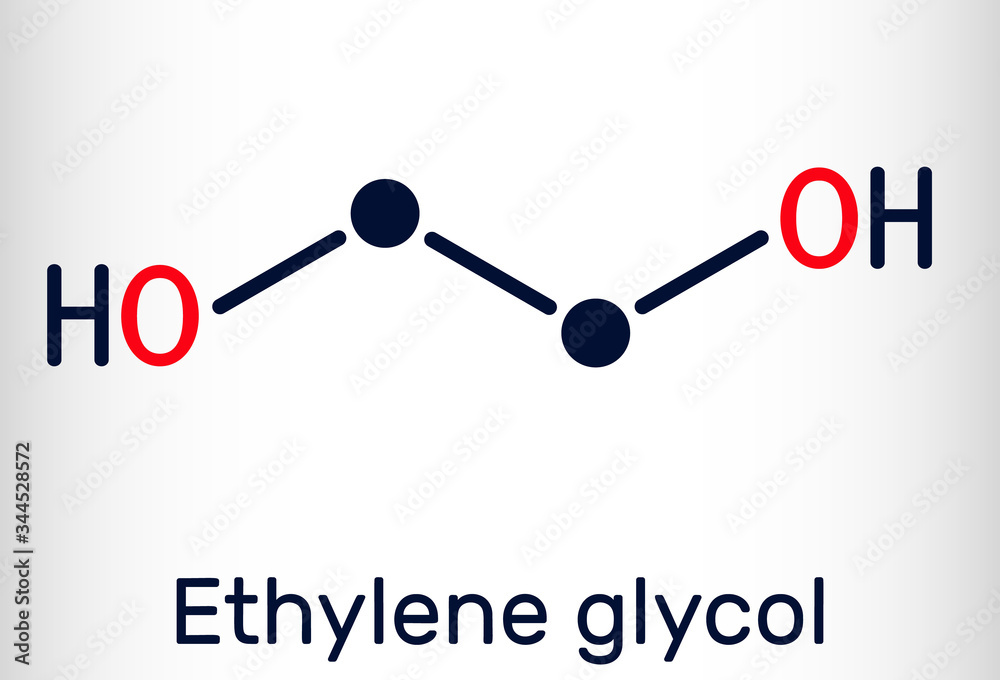
Antifreeze Chemical Formula . It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. It can cause acute and chronic health effects by ingestion, inhalation, or dermal exposure. Its most common use is as an automotive antifreeze. Eg is predominantly used as an antifreeze in cooling and heating systems, due to its excellent ability to lower the freezing point. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. A 1:1 solution of ethylene glycol and.
from stock.adobe.com
Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. Its most common use is as an automotive antifreeze. Eg is predominantly used as an antifreeze in cooling and heating systems, due to its excellent ability to lower the freezing point. It can cause acute and chronic health effects by ingestion, inhalation, or dermal exposure. Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). A 1:1 solution of ethylene glycol and. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent.
Ethylene glycol, diol, C2H6O2 molecule. It is used for manufacture of
Antifreeze Chemical Formula A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Its most common use is as an automotive antifreeze. Eg is predominantly used as an antifreeze in cooling and heating systems, due to its excellent ability to lower the freezing point. Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. It can cause acute and chronic health effects by ingestion, inhalation, or dermal exposure. Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. A 1:1 solution of ethylene glycol and. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2.
From stock.adobe.com
Ethylene glycol, diol, C2H6O2 molecule. It is used for manufacture of Antifreeze Chemical Formula Its most common use is as an automotive antifreeze. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol is a dihydroxy alcohol with. Antifreeze Chemical Formula.
From www.alamy.com
Ethylene glycol car antifreeze and polyester building block molecule Antifreeze Chemical Formula Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. It can cause acute and. Antifreeze Chemical Formula.
From www.nrao.edu
Scientists Discover Antifreeze in Space Antifreeze Chemical Formula It can cause acute and chronic health effects by ingestion, inhalation, or dermal exposure. Its most common use is as an automotive antifreeze. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Eg is predominantly used as an antifreeze in. Antifreeze Chemical Formula.
From www.alamy.com
Ethylene glycol car antifreeze and polyester building block molecule Antifreeze Chemical Formula Its most common use is as an automotive antifreeze. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Eg is predominantly used as an antifreeze in cooling and heating systems, due to its excellent ability to lower the. Antifreeze Chemical Formula.
From www.saubhaya.com
Chemical Makeup Of Antifreeze Saubhaya Makeup Antifreeze Chemical Formula Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. A 1:1 solution of ethylene glycol and. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Its most common use is as an automotive antifreeze. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. Eg is predominantly used as. Antifreeze Chemical Formula.
From www.saubhaya.com
Chemical Makeup Of Antifreeze Saubhaya Makeup Antifreeze Chemical Formula Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. Eg is predominantly used as an. Antifreeze Chemical Formula.
From www.dreamstime.com
Ethylene Glycol, Diol Molecule. it is Used for Manufacture of Polyester Antifreeze Chemical Formula Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. A 1:1 solution of ethylene glycol and. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. Ethylene glycol is a chemical used as. Antifreeze Chemical Formula.
From ethylene-glycol.weebly.com
Info antifreeze Antifreeze Chemical Formula Its most common use is as an automotive antifreeze. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. A 1:1 solution of ethylene glycol and.. Antifreeze Chemical Formula.
From www.alamy.com
Ethylene glycol car antifreeze and polyester building block molecule Antifreeze Chemical Formula It can cause acute and chronic health effects by ingestion, inhalation, or dermal exposure. Eg is predominantly used as an antifreeze in cooling and heating systems, due to its excellent ability to lower the freezing point. A 1:1 solution of ethylene glycol and. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. It is used as. Antifreeze Chemical Formula.
From mybios.me
Chemical Formula Of Mercial Antifreeze Bios Pics Antifreeze Chemical Formula Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation.. Antifreeze Chemical Formula.
From www.dreamstime.com
Set Antifreeze Canister, Oil Price Increase and Chemical Formula Antifreeze Chemical Formula Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. A 1:1 solution of ethylene glycol and. Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Eg is predominantly used as an antifreeze in cooling and heating systems, due to. Antifreeze Chemical Formula.
From www.saubhaya.com
Chemical Makeup Of Antifreeze Saubhaya Makeup Antifreeze Chemical Formula Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. Eg is predominantly used as an antifreeze in cooling and heating systems, due to its excellent ability to lower the freezing point. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Antifreeze, any substance that lowers the freezing point of water, protecting. Antifreeze Chemical Formula.
From www.alamy.com
Ethylene glycol, diol molecule. It is used for manufacture of polyester Antifreeze Chemical Formula Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. A 1:1 solution of ethylene glycol and. Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. Ethylene glycol is a clear, sweet, slightly. Antifreeze Chemical Formula.
From www.alamy.com
Ethylene glycol, diol, C2H6O2 molecule. It is used for manufacture of Antifreeze Chemical Formula Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. Its most common use is as an automotive antifreeze. Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. It can cause acute and chronic health. Antifreeze Chemical Formula.
From byjus.com
An organic compound ‘X’ which is sometimes used as an antifreeze, has Antifreeze Chemical Formula Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. Its most common use is as an automotive antifreeze. Eg is predominantly used as an antifreeze in cooling and heating systems, due to its. Antifreeze Chemical Formula.
From www.scribd.com
Antifreeze Coolant PDF Chemical Substances Chemistry Antifreeze Chemical Formula Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Its most common use is as an automotive antifreeze. It can cause acute and chronic health effects by ingestion, inhalation, or dermal exposure. Ethylene glycol is a dihydroxy alcohol. Antifreeze Chemical Formula.
From exokpvyfw.blob.core.windows.net
Antifreeze Coolant Chemical at Eric Vines blog Antifreeze Chemical Formula A 1:1 solution of ethylene glycol and. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. Ethylene glycol is a clear, sweet, slightly viscous liquid. Antifreeze Chemical Formula.
From www.saubhaya.com
Chemical Makeup Of Coolant Saubhaya Makeup Antifreeze Chemical Formula Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. A 1:1 solution of ethylene glycol and. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Ethylene glycol. Antifreeze Chemical Formula.
From www.numerade.com
Antifreeze Research ethylene glycol, an antifreezecoolant, to learn Antifreeze Chemical Formula It can cause acute and chronic health effects by ingestion, inhalation, or dermal exposure. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. Its most common use is as an automotive antifreeze. A. Antifreeze Chemical Formula.
From www.animalia-life.club
Ethylene Glycol Structural Formula Antifreeze Chemical Formula Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. It can cause acute and chronic health effects by ingestion, inhalation, or dermal exposure. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. Ethylene glycol is a clear, sweet, slightly. Antifreeze Chemical Formula.
From mybios.me
Chemical Formula Of Mercial Antifreeze Bios Pics Antifreeze Chemical Formula Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. Eg is predominantly used as an antifreeze in cooling and heating systems, due to its excellent ability to lower the freezing point. It can cause acute and chronic health effects by ingestion, inhalation, or dermal exposure. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils. Antifreeze Chemical Formula.
From mybios.me
Chemical Formula Of Mercial Antifreeze Bios Pics Antifreeze Chemical Formula Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. Its most common use is as an automotive antifreeze. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. Eg is predominantly used as an antifreeze in cooling and heating systems, due to its excellent ability to. Antifreeze Chemical Formula.
From cartoondealer.com
Ethylene Glycol Molecular Structure, 3d Model Molecule, Antifreeze Antifreeze Chemical Formula A 1:1 solution of ethylene glycol and. Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. Its most common use is as an automotive antifreeze. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils. Antifreeze Chemical Formula.
From mybios.me
Chemical Formula Of Mercial Antifreeze Bios Pics Antifreeze Chemical Formula Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Eg is. Antifreeze Chemical Formula.
From www.saubhaya.com
Chemical Makeup Of Antifreeze Saubhaya Makeup Antifreeze Chemical Formula Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. Its most common use is as an automotive antifreeze. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at. Antifreeze Chemical Formula.
From www.dreamstime.com
Set Antifreeze Canister, Chemical Formula Consisting of Benzene Rings Antifreeze Chemical Formula It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. It can cause acute and chronic health effects by ingestion, inhalation, or dermal exposure. Eg is predominantly used as an antifreeze in cooling and heating systems, due to its excellent ability to lower the. Antifreeze Chemical Formula.
From www.alamy.com
3D image of Ethylene glycol skeletal formula molecular chemical Antifreeze Chemical Formula It can cause acute and chronic health effects by ingestion, inhalation, or dermal exposure. Its most common use is as an automotive antifreeze. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Eg is predominantly used as an antifreeze in cooling and heating systems, due to its excellent ability to lower the freezing point.. Antifreeze Chemical Formula.
From www.dreamstime.com
Set Chemical Formula Consisting of Benzene Rings, Antifreeze Canister Antifreeze Chemical Formula Its most common use is as an automotive antifreeze. Eg is predominantly used as an antifreeze in cooling and heating systems, due to its excellent ability to lower the freezing point. A 1:1 solution of ethylene glycol and. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). It is used as an antifreeze,. Antifreeze Chemical Formula.
From www.arrowchem.com
ANTIFREEZE is an ethylene glycol based engine coolant concentrate Antifreeze Chemical Formula Its most common use is as an automotive antifreeze. Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at. Antifreeze Chemical Formula.
From stock.adobe.com
ethylene glycol molecular structure, 3d model molecule, antifreeze Antifreeze Chemical Formula Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). A 1:1 solution of ethylene glycol and. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. Its most common use. Antifreeze Chemical Formula.
From www.dreamstime.com
Set Antifreeze Canister, Chemical Formula Consisting of Benzene Rings Antifreeze Chemical Formula Ethylene glycol is a dihydroxy alcohol with the chemical formula c2h6o2. A 1:1 solution of ethylene glycol and. Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Ethylene glycol is a chemical used as. Antifreeze Chemical Formula.
From mybios.me
Chemical Formula Of Mercial Antifreeze Bios Pics Antifreeze Chemical Formula Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). A 1:1 solution of ethylene glycol and. Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. Eg is predominantly used. Antifreeze Chemical Formula.
From www.alamy.com
Ethylene glycol car antifreeze and polyester building block molecule Antifreeze Chemical Formula Antifreeze, any substance that lowers the freezing point of water, protecting a system from the ill effects of ice formation. Its most common use is as an automotive antifreeze. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. Ethylene glycol is a clear, sweet, slightly viscous liquid that boils at 198 °c (388.4 °f). A 1:1. Antifreeze Chemical Formula.
From mungfali.com
Ethylene Glycol Chemical Formula Antifreeze Chemical Formula It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. It can cause acute and chronic health effects by ingestion, inhalation, or dermal exposure. Its most common use is as an automotive antifreeze. Eg is predominantly used as an antifreeze in cooling and heating systems, due to its excellent ability to lower the freezing point.. Antifreeze Chemical Formula.
From mybios.me
Chemical Formula Of Mercial Antifreeze Bios Pics Antifreeze Chemical Formula Eg is predominantly used as an antifreeze in cooling and heating systems, due to its excellent ability to lower the freezing point. Ethylene glycol is a chemical used as antifreeze and has the formula c2h6o2. It is used as an antifreeze, a raw material for polyester, and a dehydrating agent. Its most common use is as an automotive antifreeze. Antifreeze,. Antifreeze Chemical Formula.