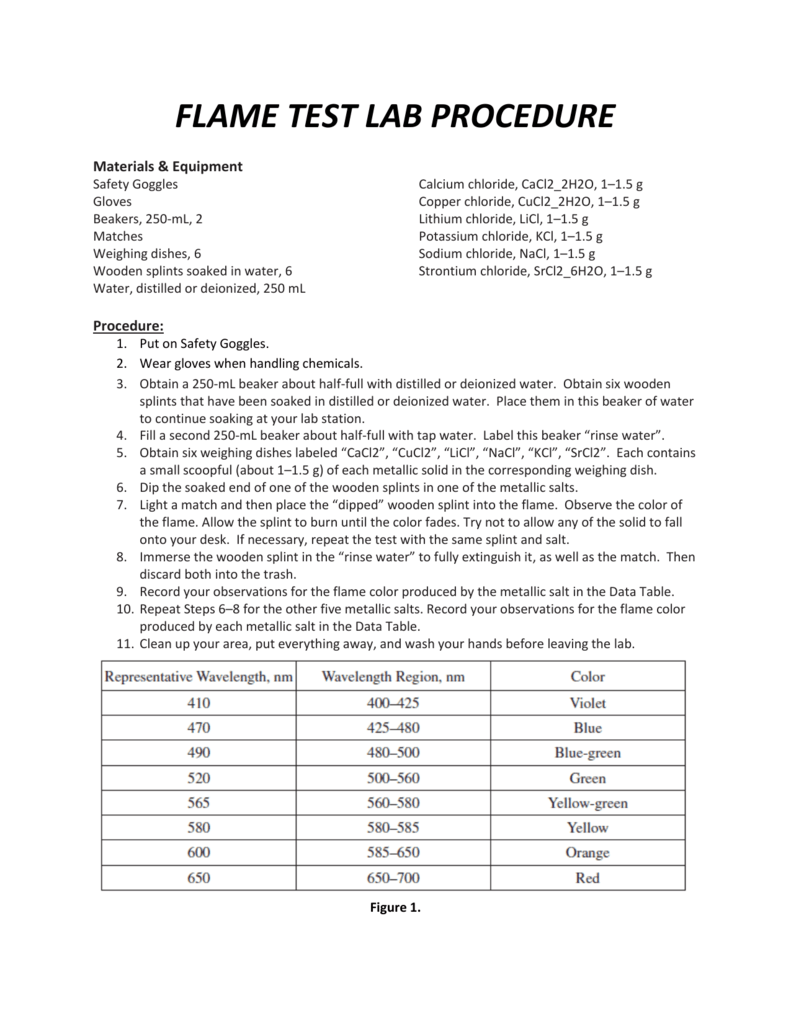
Flame Test Lab Analysis Questions . A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. Could flame tests be useful in identifying the identity of elements? For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table below. Observe the flame test for seven different metallic ions. in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. For your reference, a table 1 from your lab. Identify an unknown metallic element, using the. in this lab, you will perform flame tests of several different metal cations. what is the flame test?
from learningmedialionel.z13.web.core.windows.net
in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. Identify an unknown metallic element, using the. Observe the flame test for seven different metallic ions. in this lab, you will perform flame tests of several different metal cations. For your reference, a table 1 from your lab. A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table below. what is the flame test? Could flame tests be useful in identifying the identity of elements?
Flame Test Lab Worksheet
Flame Test Lab Analysis Questions A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table below. Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. what is the flame test? Identify an unknown metallic element, using the. in this lab, you will perform flame tests of several different metal cations. For your reference, a table 1 from your lab. Observe the flame test for seven different metallic ions. Could flame tests be useful in identifying the identity of elements?
From www.docsity.com
Flame test lab part 2 Study Guides, Projects, Research Chemistry Flame Test Lab Analysis Questions For your reference, a table 1 from your lab. Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table. Flame Test Lab Analysis Questions.
From myans.bhantedhammika.net
Flame Test Lab Report Flame Test Lab Analysis Questions A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. in this lab, you will perform flame tests of several different metal cations. For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table below. Compare and contrast the early atomic theories. Flame Test Lab Analysis Questions.
From www.studypool.com
SOLUTION Lab 2 measurement flame test Studypool Flame Test Lab Analysis Questions Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table below. A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. what is the flame test? For your. Flame Test Lab Analysis Questions.
From studylib.net
LAB Flame test Flame Test Lab Analysis Questions in this lab, you will perform flame tests of several different metal cations. what is the flame test? A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. Observe the flame test for seven different metallic ions. For each metal cation flame test performed, determine the wavelength corresponding to. Flame Test Lab Analysis Questions.
From studylib.net
Flame Test Lab Flame Test Lab Analysis Questions what is the flame test? For your reference, a table 1 from your lab. Observe the flame test for seven different metallic ions. Identify an unknown metallic element, using the. in this lab, you will perform flame tests of several different metal cations. A flame test is a qualitative analysis used by the chemist to identify the metal. Flame Test Lab Analysis Questions.
From learningmedialionel.z13.web.core.windows.net
Flame Test Lab Worksheet Flame Test Lab Analysis Questions in this lab, you will perform flame tests of several different metal cations. Could flame tests be useful in identifying the identity of elements? Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. Observe the flame test for seven different metallic ions. in this experiment, you will be performing qualitative identification of alkali, alkaline,. Flame Test Lab Analysis Questions.
From studylib.net
Flame Test Lab Flame Test Lab Analysis Questions Could flame tests be useful in identifying the identity of elements? A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table below. in this lab, you will perform flame tests of. Flame Test Lab Analysis Questions.
From www.tes.com
Flame Test Lab Teaching Resources Flame Test Lab Analysis Questions in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. For your reference, a table 1 from your lab. what is the flame test? in this lab, you will perform flame tests of. Flame Test Lab Analysis Questions.
From studylib.net
FLAME TEST LAB Flame Test Lab Analysis Questions Observe the flame test for seven different metallic ions. For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table below. Could flame tests be useful in identifying the identity of elements? A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. . Flame Test Lab Analysis Questions.
From athensmutualaid.net
Flame Test Lab Questions Answer Key › Athens Mutual Student Corner Flame Test Lab Analysis Questions Observe the flame test for seven different metallic ions. what is the flame test? Could flame tests be useful in identifying the identity of elements? Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. Identify an unknown metallic element, using the. A flame test is a qualitative analysis used by the chemist to identify the. Flame Test Lab Analysis Questions.
From studylib.net
Flame Tests Lab Flame Test Lab Analysis Questions Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. what is the flame test? Could flame tests be useful in identifying the identity of elements? For your reference, a table 1 from your lab. Observe the. Flame Test Lab Analysis Questions.
From ctdatmanshah.blogspot.com
Lab 11 Flame Test Lab Flame Test Lab Analysis Questions in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. Identify an unknown metallic element, using the. Could flame tests be useful in identifying the identity of elements? Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. For each metal cation flame test performed, determine the wavelength corresponding to the. Flame Test Lab Analysis Questions.
From hightechhigh-faithsdp.weebly.com
Flame Test Lab Faith's DP Flame Test Lab Analysis Questions Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table below. A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. in this experiment, you will be performing. Flame Test Lab Analysis Questions.
From www.pinterest.com
Lab Activity Identifying Elements Using Flame Tests Lab activities Flame Test Lab Analysis Questions Could flame tests be useful in identifying the identity of elements? Identify an unknown metallic element, using the. Observe the flame test for seven different metallic ions. in this lab, you will perform flame tests of several different metal cations. For your reference, a table 1 from your lab. Compare and contrast the early atomic theories of dalton, thomson,. Flame Test Lab Analysis Questions.
From ar.inspiredpencil.com
Flame Test Lab Answer Key Flame Test Lab Analysis Questions For your reference, a table 1 from your lab. Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. Could flame tests be useful in identifying the identity of elements? For each metal cation flame test performed, determine. Flame Test Lab Analysis Questions.
From studylib.net
Flame Test Lab Questions Answer Key (1) Flame Test Lab Analysis Questions Could flame tests be useful in identifying the identity of elements? what is the flame test? Identify an unknown metallic element, using the. Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. For your reference, a table 1 from. Flame Test Lab Analysis Questions.
From www.scribd.com
Lab Flame Test Handout Chem Emission Spectrum Scientific Techniques Flame Test Lab Analysis Questions For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table below. what is the flame test? Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. Observe the flame test for seven different metallic ions. A flame test is a qualitative analysis used by the chemist to. Flame Test Lab Analysis Questions.
From athensmutualaid.net
Flame Test Lab Pdf › Athens Mutual Student Corner Flame Test Lab Analysis Questions in this lab, you will perform flame tests of several different metal cations. Observe the flame test for seven different metallic ions. in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. For your reference, a table 1 from your lab. what is the flame test? Could flame tests be useful in. Flame Test Lab Analysis Questions.
From www.animalia-life.club
Flame Test Lab Results Flame Test Lab Analysis Questions Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. what is the flame test? Could flame tests be useful in identifying the identity of elements? For your reference, a table 1 from your lab. Observe the. Flame Test Lab Analysis Questions.
From sciencelessonsthatrock.com
Chemistry Flame Test Lab Science Lessons That Rock Flame Test Lab Analysis Questions Observe the flame test for seven different metallic ions. Identify an unknown metallic element, using the. what is the flame test? A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. For your reference, a table 1 from your lab. Could flame tests be useful in identifying the identity of. Flame Test Lab Analysis Questions.
From www.youtube.com
Flame Test Lab Calculations YouTube Flame Test Lab Analysis Questions For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table below. Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. Identify an unknown metallic element, using the. in this lab, you will perform flame tests of several different metal cations. Could flame tests be useful in. Flame Test Lab Analysis Questions.
From studylib.net
Flame Test Lab Flame Test Lab Analysis Questions in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. Identify an unknown metallic element, using the. A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. For your reference, a table 1 from your lab. what is the flame test? Could flame tests. Flame Test Lab Analysis Questions.
From www.scribd.com
Flame Test Lab Example Emission Spectrum Atoms Flame Test Lab Analysis Questions Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. in this lab, you will perform flame tests of several different metal cations. For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table below. For your reference, a table 1 from your lab. A flame test is. Flame Test Lab Analysis Questions.
From studylib.net
Flame Test Lab Report Flame Test Lab Analysis Questions in this lab, you will perform flame tests of several different metal cations. Could flame tests be useful in identifying the identity of elements? Observe the flame test for seven different metallic ions. Identify an unknown metallic element, using the. what is the flame test? in this experiment, you will be performing qualitative identification of alkali, alkaline,. Flame Test Lab Analysis Questions.
From dxoqbloyt.blob.core.windows.net
Flame Test Lab The Identification Of An Element Answers at Linda Flame Test Lab Analysis Questions Could flame tests be useful in identifying the identity of elements? For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table below. in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. A flame test is a qualitative analysis used by the chemist to identify. Flame Test Lab Analysis Questions.
From studylib.net
Flame Tests Lab Flame Test Lab Analysis Questions For your reference, a table 1 from your lab. Could flame tests be useful in identifying the identity of elements? in this lab, you will perform flame tests of several different metal cations. in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. A flame test is a qualitative analysis used by the. Flame Test Lab Analysis Questions.
From studylib.net
2nd 6wk Flame Test template Flame Test Lab Analysis Questions For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table below. Could flame tests be useful in identifying the identity of elements? A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. in this experiment, you will be performing qualitative identification. Flame Test Lab Analysis Questions.
From studylib.net
14 Flame Test lab fy11 Flame Test Lab Analysis Questions Identify an unknown metallic element, using the. in this lab, you will perform flame tests of several different metal cations. in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. what is the flame test? Could flame tests be useful in identifying the identity of elements? Compare and contrast the early atomic. Flame Test Lab Analysis Questions.
From www.docsity.com
Flame Test Lab Worksheet Activity with Answers Key Exercises Flame Test Lab Analysis Questions Observe the flame test for seven different metallic ions. what is the flame test? Identify an unknown metallic element, using the. A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. For each metal cation flame test performed, determine the wavelength corresponding to the observed flame color from the table. Flame Test Lab Analysis Questions.
From slideplayer.com
Flame Test Lab. ppt download Flame Test Lab Analysis Questions what is the flame test? For your reference, a table 1 from your lab. in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. Identify an unknown metallic element, using the. in this lab, you will perform flame tests of several different metal cations. Observe the flame test for seven different metallic. Flame Test Lab Analysis Questions.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Flame Test Lab Analysis Questions Identify an unknown metallic element, using the. in this lab, you will perform flame tests of several different metal cations. A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. For your reference, a table 1 from your lab. what is the flame test? Observe the flame test for. Flame Test Lab Analysis Questions.
From studylib.net
Flame Test Lab Flame Test Lab Analysis Questions what is the flame test? in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. Could flame tests be useful in identifying the identity of elements? Identify an unknown metallic element, using the. Observe the flame test for seven different metallic ions. A flame test is a qualitative analysis used by the chemist. Flame Test Lab Analysis Questions.
From www.flipsnack.com
Flame Test Lab by Jesus Sanchez Flipsnack Flame Test Lab Analysis Questions Identify an unknown metallic element, using the. Observe the flame test for seven different metallic ions. in this lab, you will perform flame tests of several different metal cations. Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. For your reference, a table 1 from your lab. what is the flame test? in. Flame Test Lab Analysis Questions.
From www.stuvia.com
FLAME TESTS LAB QUESTIONS AND ANSWERS FLAME Stuvia US Flame Test Lab Analysis Questions what is the flame test? in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. in this lab, you will perform flame tests of several different metal cations. Compare and contrast the early atomic theories of dalton, thomson, rutherford, and millikan. A flame test is a qualitative analysis used by the chemist. Flame Test Lab Analysis Questions.
From athensmutualaid.net
Chemistry Flame Test Lab Answer Key Pdf › Athens Mutual Student Corner Flame Test Lab Analysis Questions For your reference, a table 1 from your lab. A flame test is a qualitative analysis used by the chemist to identify the metal and metalloid ion in. in this experiment, you will be performing qualitative identification of alkali, alkaline, and transition metals. what is the flame test? Identify an unknown metallic element, using the. For each metal. Flame Test Lab Analysis Questions.