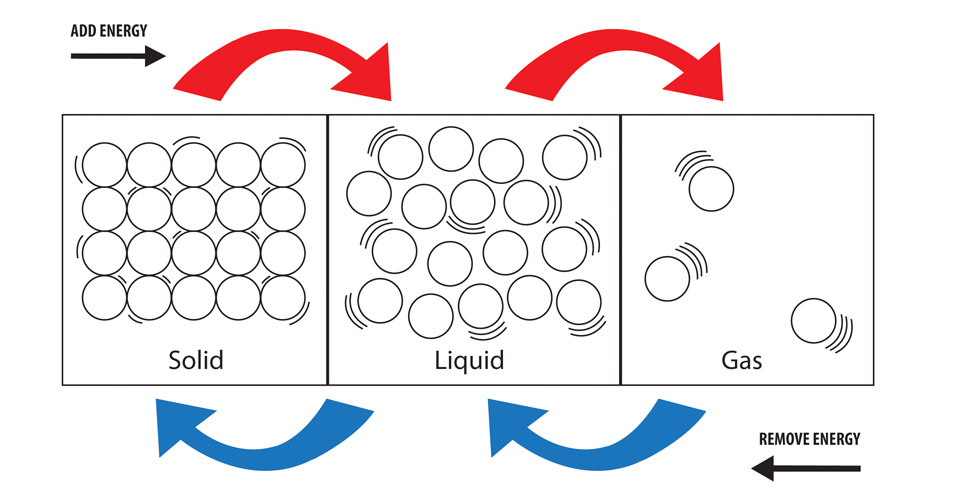
What Happens To Potential Energy When Ice Melts . in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. Heat is transferred from the soda to the ice for melting. Indeed, this is the only increase in energy, since. when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system (the ice), causing the ice to melt and your. At a higher temperature, more. the ice cubes are at the melting temperature of 0 °c °c. eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules. yes, potential energy increases with increasing temperature for at least the following three reasons: this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: when ice or any other solid melts, its potential energy increases.
from socratic.org
yes, potential energy increases with increasing temperature for at least the following three reasons: this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system (the ice), causing the ice to melt and your. the ice cubes are at the melting temperature of 0 °c °c. Heat is transferred from the soda to the ice for melting. At a higher temperature, more. when ice or any other solid melts, its potential energy increases. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. Indeed, this is the only increase in energy, since. eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules.
What happens to the energy of its molecules as ice melts into
What Happens To Potential Energy When Ice Melts yes, potential energy increases with increasing temperature for at least the following three reasons: in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. when ice or any other solid melts, its potential energy increases. the ice cubes are at the melting temperature of 0 °c °c. At a higher temperature, more. Heat is transferred from the soda to the ice for melting. when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system (the ice), causing the ice to melt and your. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Indeed, this is the only increase in energy, since. yes, potential energy increases with increasing temperature for at least the following three reasons: eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules.
From www.slideserve.com
PPT What happens when ICE melts? PowerPoint Presentation, free What Happens To Potential Energy When Ice Melts yes, potential energy increases with increasing temperature for at least the following three reasons: when ice or any other solid melts, its potential energy increases. eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules. this plot of temperature shows what happens. What Happens To Potential Energy When Ice Melts.
From www.lihpao.com
What Melts Ice the Fastest? Exploring Different Methods in a Science What Happens To Potential Energy When Ice Melts when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system (the ice), causing the ice to melt and your. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. At a higher temperature, more. the ice cubes are at. What Happens To Potential Energy When Ice Melts.
From classroom.synonym.com
What Happens When Ice Is Added to Hot Water and How Will the Energy What Happens To Potential Energy When Ice Melts yes, potential energy increases with increasing temperature for at least the following three reasons: eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules. the ice cubes are at the melting temperature of 0 °c °c. in the case of ice melting. What Happens To Potential Energy When Ice Melts.
From courses.lumenlearning.com
Phase Change and Latent Heat Boundless Physics What Happens To Potential Energy When Ice Melts when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system (the ice), causing the ice to melt and your. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. yes, potential energy increases with increasing temperature for at least. What Happens To Potential Energy When Ice Melts.
From www.vectorstock.com
Diagram showing how ice melts Royalty Free Vector Image What Happens To Potential Energy When Ice Melts the ice cubes are at the melting temperature of 0 °c °c. when ice or any other solid melts, its potential energy increases. Heat is transferred from the soda to the ice for melting. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is. What Happens To Potential Energy When Ice Melts.
From briesnitzhnschematic.z14.web.core.windows.net
What Happens When Thermal Energy Increases What Happens To Potential Energy When Ice Melts when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system (the ice), causing the ice to melt and your. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. What Happens To Potential Energy When Ice Melts.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens To Potential Energy When Ice Melts Indeed, this is the only increase in energy, since. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: yes, potential energy increases with increasing temperature for at least the following three reasons: in the case of ice melting. What Happens To Potential Energy When Ice Melts.
From www.haikudeck.com
Ice Melt by Sean Mulhern What Happens To Potential Energy When Ice Melts when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system (the ice), causing the ice to melt and your. when ice or any other solid melts, its potential energy increases. Indeed, this is the only increase in energy, since. Heat is transferred from the soda to the. What Happens To Potential Energy When Ice Melts.
From sciencing.com
Does Energy Increase in a Drink When Ice Melts? Sciencing What Happens To Potential Energy When Ice Melts yes, potential energy increases with increasing temperature for at least the following three reasons: when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system (the ice), causing the ice to melt and your. the ice cubes are at the melting temperature of 0 °c °c. . What Happens To Potential Energy When Ice Melts.
From www.slideserve.com
PPT Melting Ice Cubes aka. Thermodynamics and Heat Transfer What Happens To Potential Energy When Ice Melts eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules. Heat is transferred from the soda to the ice for melting. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. when ice or any other. What Happens To Potential Energy When Ice Melts.
From www.slideserve.com
PPT Pascal’s Principle PowerPoint Presentation, free download ID What Happens To Potential Energy When Ice Melts in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. yes, potential energy increases with increasing temperature for at least the following three reasons: At a higher temperature, more. when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system. What Happens To Potential Energy When Ice Melts.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens To Potential Energy When Ice Melts when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system (the ice), causing the ice to melt and your. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate:. What Happens To Potential Energy When Ice Melts.
From studylib.net
What Happens to Molecules When a Substance Melts? Ice Liquid What Happens To Potential Energy When Ice Melts when ice or any other solid melts, its potential energy increases. At a higher temperature, more. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Heat is transferred from the soda to the ice for melting. in the. What Happens To Potential Energy When Ice Melts.
From huntingwaterfalls.com
What Exactly Happens When an Ice Cube Melts? (Simple Science Explained) What Happens To Potential Energy When Ice Melts Indeed, this is the only increase in energy, since. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system. What Happens To Potential Energy When Ice Melts.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens To Potential Energy When Ice Melts the ice cubes are at the melting temperature of 0 °c °c. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. Heat is transferred from the soda to the ice for melting. this plot of temperature shows what happens to a 75 g sample of ice initially at 1. What Happens To Potential Energy When Ice Melts.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens To Potential Energy When Ice Melts when ice or any other solid melts, its potential energy increases. yes, potential energy increases with increasing temperature for at least the following three reasons: eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules. Indeed, this is the only increase in energy,. What Happens To Potential Energy When Ice Melts.
From ian.umces.edu
Experiment Demonstrating Melting Ice Media Library Integration and What Happens To Potential Energy When Ice Melts in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. Heat is transferred from the soda to the ice for melting. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: yes,. What Happens To Potential Energy When Ice Melts.
From www.pbs.org
PhaseChange Materials Exploit Thermodynamics to Save Energy What Happens To Potential Energy When Ice Melts eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules. At a higher temperature, more. when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system (the ice), causing the ice to melt and. What Happens To Potential Energy When Ice Melts.
From www.youtube.com
What happens to water level, when ice melts। NEET/JEE। Physics।Fluid What Happens To Potential Energy When Ice Melts Heat is transferred from the soda to the ice for melting. eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules. yes, potential energy increases with increasing temperature for at least the following three reasons: At a higher temperature, more. the ice cubes. What Happens To Potential Energy When Ice Melts.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens To Potential Energy When Ice Melts when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system (the ice), causing the ice to melt and your. At a higher temperature, more. Heat is transferred from the soda to the ice for melting. when ice or any other solid melts, its potential energy increases. . What Happens To Potential Energy When Ice Melts.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens To Potential Energy When Ice Melts eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules. when ice or any other solid melts, its potential energy increases. At a higher temperature, more. the ice cubes are at the melting temperature of 0 °c °c. Heat is transferred from the. What Happens To Potential Energy When Ice Melts.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens To Potential Energy When Ice Melts when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system (the ice), causing the ice to melt and your. yes, potential energy increases with increasing temperature for at least the following three reasons: the ice cubes are at the melting temperature of 0 °c °c. . What Happens To Potential Energy When Ice Melts.
From slideplayer.com
Ch 2 Matter and Energy When ice melts what happens to its chemical What Happens To Potential Energy When Ice Melts the ice cubes are at the melting temperature of 0 °c °c. yes, potential energy increases with increasing temperature for at least the following three reasons: when ice or any other solid melts, its potential energy increases. eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces. What Happens To Potential Energy When Ice Melts.
From www.britannica.com
Ice Definition, Structure, Properties, Freezing Point, & Facts What Happens To Potential Energy When Ice Melts when ice or any other solid melts, its potential energy increases. eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. this plot of temperature. What Happens To Potential Energy When Ice Melts.
From www.slideserve.com
PPT Chapter 17 Free Energy and Thermodynamics PowerPoint Presentation What Happens To Potential Energy When Ice Melts Indeed, this is the only increase in energy, since. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. the ice cubes. What Happens To Potential Energy When Ice Melts.
From sciencing.com
What Happens to the Temperature of Ice As it Melts? Sciencing What Happens To Potential Energy When Ice Melts Heat is transferred from the soda to the ice for melting. this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: Indeed, this is the only increase in energy, since. when you hold an ice cube in your hand, heat. What Happens To Potential Energy When Ice Melts.
From www.scienceabc.com
What Is Gibbs Free Energy? What Is Its Equation? » ScienceABC What Happens To Potential Energy When Ice Melts eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. when you hold an ice cube in your hand, heat from the surroundings (including your hand). What Happens To Potential Energy When Ice Melts.
From www.scienceabc.com
What Is The First Law Of Thermodynamics? » ScienceABC What Happens To Potential Energy When Ice Melts eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules. At a higher temperature, more. the ice cubes are at the melting temperature of 0 °c °c. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal. What Happens To Potential Energy When Ice Melts.
From www.slideserve.com
PPT Melting ice PowerPoint Presentation, free download ID1452801 What Happens To Potential Energy When Ice Melts eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules. yes, potential energy increases with increasing temperature for at least the following three reasons: Indeed, this is the only increase in energy, since. Heat is transferred from the soda to the ice for melting.. What Happens To Potential Energy When Ice Melts.
From www.researchgate.net
Equilibrium coexistence of ice and water. Illustration of potential What Happens To Potential Energy When Ice Melts Indeed, this is the only increase in energy, since. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. Heat is transferred from the soda to the ice for melting. the ice cubes are at the melting temperature of 0 °c °c. when ice or any other solid melts, its. What Happens To Potential Energy When Ice Melts.
From apollo.lsc.vsc.edu
Latent Heat of evaporation, fusion, and freezing What Happens To Potential Energy When Ice Melts Indeed, this is the only increase in energy, since. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. when ice or any other solid melts, its potential energy increases. At a higher temperature, more. Heat is transferred from the soda to the ice for melting. this plot of temperature. What Happens To Potential Energy When Ice Melts.
From ar.inspiredpencil.com
Melting Point Of Ice What Happens To Potential Energy When Ice Melts Indeed, this is the only increase in energy, since. eventually, when the ice has warmed to 0°c, the added energy will start to overcome the attractive intermolecular forces that hold the water molecules. in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. yes, potential energy increases with increasing temperature. What Happens To Potential Energy When Ice Melts.
From socratic.org
What happens to the energy of its molecules as ice melts into What Happens To Potential Energy When Ice Melts this plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and −23°c as heat is added at a constant rate: the ice cubes are at the melting temperature of 0 °c °c. At a higher temperature, more. when ice or any other solid melts, its potential energy increases. . What Happens To Potential Energy When Ice Melts.
From punchlistzero.com
Specific Heat of Ice In Various Units, vs. Water, Ice's Thermal What Happens To Potential Energy When Ice Melts in the case of ice melting under atmospheric pressure, the volume contraction means that the internal energy. yes, potential energy increases with increasing temperature for at least the following three reasons: when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system (the ice), causing the ice. What Happens To Potential Energy When Ice Melts.
From philschatz.com
Phase Change and Latent Heat · Physics What Happens To Potential Energy When Ice Melts when ice or any other solid melts, its potential energy increases. when you hold an ice cube in your hand, heat from the surroundings (including your hand) is transferred to the system (the ice), causing the ice to melt and your. the ice cubes are at the melting temperature of 0 °c °c. Indeed, this is the. What Happens To Potential Energy When Ice Melts.