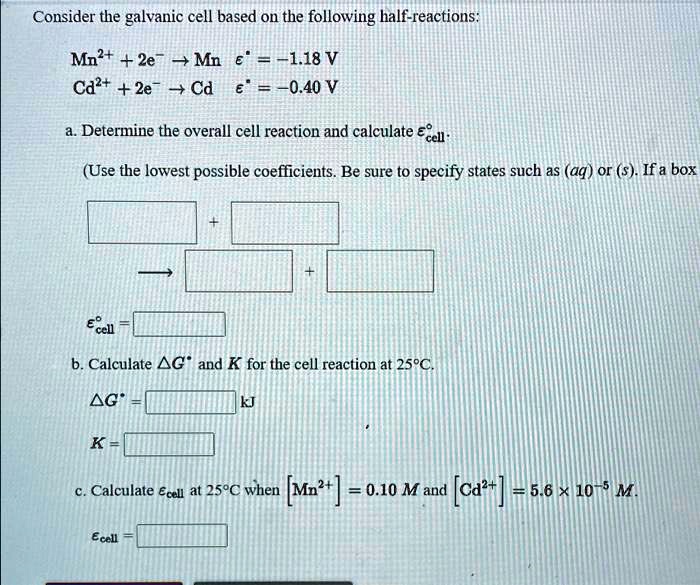
A Galvanic Cell Is Based On The Following Half-Reactions At 25 . Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3.
from www.numerade.com
Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77.
SOLVED Consider the galvanic cell based on the following half
A Galvanic Cell Is Based On The Following Half-Reactions At 25 Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,.
From www.numerade.com
SOLVED Describe completely the galvanic cell based on the following A Galvanic Cell Is Based On The Following Half-Reactions At 25 Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVED Give the balanced cell equation and determine for the galvanic A Galvanic Cell Is Based On The Following Half-Reactions At 25 Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.bartleby.com
Answered A chemist designs a galvanic cell that… bartleby A Galvanic Cell Is Based On The Following Half-Reactions At 25 Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVEDConsider the galvanic cell based on the following halfreactions A Galvanic Cell Is Based On The Following Half-Reactions At 25 A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVED Consider the galvanic cell based on the following half A Galvanic Cell Is Based On The Following Half-Reactions At 25 Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.chegg.com
Solved Consider the galvanic cell based on the following A Galvanic Cell Is Based On The Following Half-Reactions At 25 Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVED Suppose the galvanic cell sketched below is powered by the A Galvanic Cell Is Based On The Following Half-Reactions At 25 Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVEDSketch the galvanic cells based on the following overall A Galvanic Cell Is Based On The Following Half-Reactions At 25 Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVEDA galvanic cell is based on the following halfreactions at 25^∘ A Galvanic Cell Is Based On The Following Half-Reactions At 25 Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVED Give the balanced cell equation and determine € for the A Galvanic Cell Is Based On The Following Half-Reactions At 25 Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVEDConsider the standard galvanic cell based on the following half A Galvanic Cell Is Based On The Following Half-Reactions At 25 Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVEDSuppose the galvanic cell sketched below /s powered by (hie A Galvanic Cell Is Based On The Following Half-Reactions At 25 Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVEDA chemist designs galvanic cell that uses these two half A Galvanic Cell Is Based On The Following Half-Reactions At 25 Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVEDSketch the galvanic cells based on the following halfreactions A Galvanic Cell Is Based On The Following Half-Reactions At 25 A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.chegg.com
Solved In a galvanic cell based on the half reactions A Galvanic Cell Is Based On The Following Half-Reactions At 25 Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.coursehero.com
[Solved] Consider the galvanic cell based on the following half A Galvanic Cell Is Based On The Following Half-Reactions At 25 Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From solvedlib.com
Galvanic cell using the half reactions below What is … SolvedLib A Galvanic Cell Is Based On The Following Half-Reactions At 25 A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.answersarena.com
[Solved] Consider the galvanic cell based on the followin A Galvanic Cell Is Based On The Following Half-Reactions At 25 Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.chegg.com
Solved 80. Consider the galvanic cell based on the following A Galvanic Cell Is Based On The Following Half-Reactions At 25 Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.solutionspile.com
[Solved] Consider the galvanic cell based on the following A Galvanic Cell Is Based On The Following Half-Reactions At 25 Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVED Consider the galvanic cell based on the following half A Galvanic Cell Is Based On The Following Half-Reactions At 25 Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.coursehero.com
[Solved] Galvanic Cells Sketch the galvanic cells based on the A Galvanic Cell Is Based On The Following Half-Reactions At 25 A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVED 6 A galvanic cell is based on the following halfreactions Eq A Galvanic Cell Is Based On The Following Half-Reactions At 25 Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.chegg.com
Solved Consider the galvanic cell based on the following A Galvanic Cell Is Based On The Following Half-Reactions At 25 Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.youtube.com
Find the cell potential of a galvanic cell based on the following A Galvanic Cell Is Based On The Following Half-Reactions At 25 A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVED This question has multiple parts. Work all the parts to get the A Galvanic Cell Is Based On The Following Half-Reactions At 25 Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVEDGive the balanced cell equation and determine ℰ^∘ for the A Galvanic Cell Is Based On The Following Half-Reactions At 25 A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVEDA chemist designs a galvanic cell that uses these two half A Galvanic Cell Is Based On The Following Half-Reactions At 25 A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVED This question has multiple parts. Work all the parts to get the A Galvanic Cell Is Based On The Following Half-Reactions At 25 Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From quizlet.com
Sketch the galvanic cells based on the following halfreactio Quizlet A Galvanic Cell Is Based On The Following Half-Reactions At 25 A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVED Q3 On the Sketch of the galvanic cells based on the following A Galvanic Cell Is Based On The Following Half-Reactions At 25 A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From quizlet.com
Sketch the galvanic cells based on the following halfreacti Quizlet A Galvanic Cell Is Based On The Following Half-Reactions At 25 A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.coursehero.com
[Solved] 6. Consider the galvanic cell based on the following half A Galvanic Cell Is Based On The Following Half-Reactions At 25 Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.coursehero.com
[Solved] Consider the galvanic cell based on the following half A Galvanic Cell Is Based On The Following Half-Reactions At 25 A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A Galvanic Cell Is Based On The Following Half-Reactions At 25.
From www.numerade.com
SOLVED Consider the galvanic cell based on the following half A Galvanic Cell Is Based On The Following Half-Reactions At 25 A galvanic cell based on the spontaneous reaction between copper and silver(i) is depicted in figure 17.3. Calculate ecell for this galvanic cell when [ag+] = 2.0 m,. Ag+ (aq) + e + ag (s) e° = +0.80 v h2o2 (aq) + 2 h+ (aq) + 2 e + 2 h2o (1) e° = +1.77. A Galvanic Cell Is Based On The Following Half-Reactions At 25.