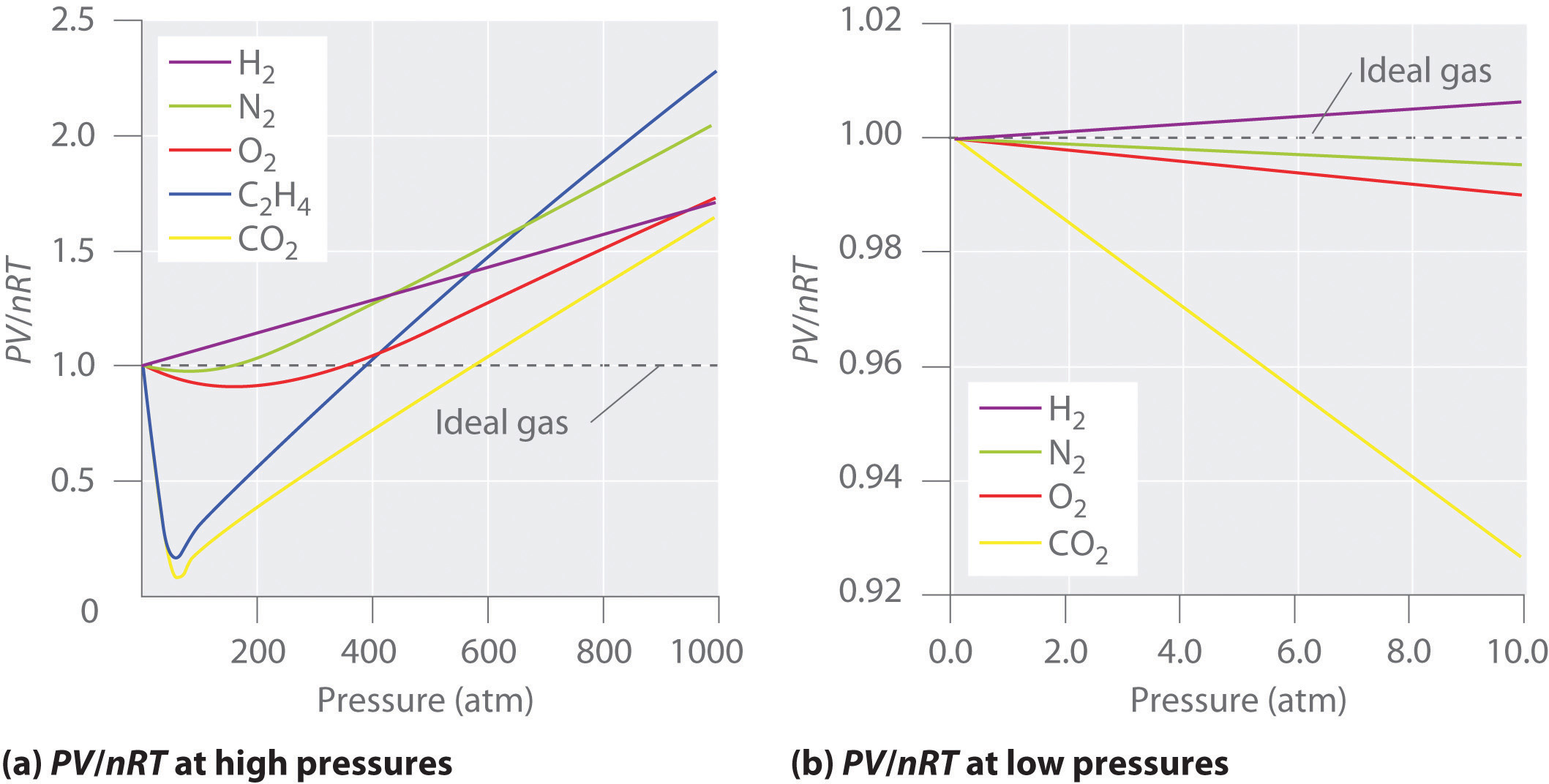
Ideal Gas And Real Gas . We also examine liquefaction, a key property of real gases that is not. In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law. Learn the key differences between ideal gas and real gas, two types of gases that have different properties and behaviours. The ideal gas law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a large number of molecules, which are in random. Fortunately, at the conditions of temperature and pressure that are normally. While the particles of an ideal gas are assumed to occupy no volume and experience no interparticle attractions, the particles of a real gas do have. Why do real gases behave so differently from ideal gases at high pressures and low temperatures? Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible volume and that intermolecular interactions are negligible—are no longer valid. Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic.
from socratic.org
In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law. Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic. Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible volume and that intermolecular interactions are negligible—are no longer valid. Why do real gases behave so differently from ideal gases at high pressures and low temperatures? We also examine liquefaction, a key property of real gases that is not. While the particles of an ideal gas are assumed to occupy no volume and experience no interparticle attractions, the particles of a real gas do have. Learn the key differences between ideal gas and real gas, two types of gases that have different properties and behaviours. Fortunately, at the conditions of temperature and pressure that are normally. The ideal gas law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a large number of molecules, which are in random.
What is the difference between an ideal gas and a real gas? Socratic
Ideal Gas And Real Gas While the particles of an ideal gas are assumed to occupy no volume and experience no interparticle attractions, the particles of a real gas do have. While the particles of an ideal gas are assumed to occupy no volume and experience no interparticle attractions, the particles of a real gas do have. Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic. Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible volume and that intermolecular interactions are negligible—are no longer valid. Learn the key differences between ideal gas and real gas, two types of gases that have different properties and behaviours. Why do real gases behave so differently from ideal gases at high pressures and low temperatures? Fortunately, at the conditions of temperature and pressure that are normally. We also examine liquefaction, a key property of real gases that is not. The ideal gas law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a large number of molecules, which are in random. In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law.
From www.geeksforgeeks.org
Difference Between Ideal Gas And Real Gas Ideal Gas And Real Gas Why do real gases behave so differently from ideal gases at high pressures and low temperatures? We also examine liquefaction, a key property of real gases that is not. The ideal gas law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a large number of molecules, which are. Ideal Gas And Real Gas.
From www.slideserve.com
PPT Unit 6 Gases & The Molecular Theory PowerPoint Ideal Gas And Real Gas In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law. Why do real gases behave so differently from ideal gases at high pressures and low temperatures? Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible volume and that. Ideal Gas And Real Gas.
From intactone.com
Important Differences Between Ideal Gas and Real Gas intactone Ideal Gas And Real Gas Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic. In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law. Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible. Ideal Gas And Real Gas.
From sciencenotes.org
Real Gas vs Ideal Gas Ideal Gas And Real Gas Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic. In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law. We also examine liquefaction, a key property of real gases that is not. Fortunately, at the conditions. Ideal Gas And Real Gas.
From www.differencebetween.com
Difference Between Ideal Gas Law and Real Gas Law Compare the Ideal Gas And Real Gas The ideal gas law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a large number of molecules, which are in random. Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic. Fortunately, at the conditions of temperature and pressure. Ideal Gas And Real Gas.
From askanydifference.com
Ideal Gas vs Real Gas Difference and Comparison Ideal Gas And Real Gas Fortunately, at the conditions of temperature and pressure that are normally. In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law. While the particles of an ideal gas are assumed to occupy no volume and experience no interparticle attractions, the particles of a real gas do. Ideal Gas And Real Gas.
From www.youtube.com
CHARACTERISTIC OF IDEAL GAS AND REAL GAS YouTube Ideal Gas And Real Gas In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law. Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic. The ideal gas law can be derived from the kinetic theory of gases and relies on the. Ideal Gas And Real Gas.
From physicsgirl.in
Ideal Gases and Real Gases A Comprehensive Overview Physics Girl Ideal Gas And Real Gas The ideal gas law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a large number of molecules, which are in random. We also examine liquefaction, a key property of real gases that is not. Why do real gases behave so differently from ideal gases at high pressures and. Ideal Gas And Real Gas.
From www.shutterstock.com
Ideal Gas Real Gas Diagram Scientific Stock Vector (Royalty Free Ideal Gas And Real Gas In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law. Fortunately, at the conditions of temperature and pressure that are normally. Learn the key differences between ideal gas and real gas, two types of gases that have different properties and behaviours. While the particles of an. Ideal Gas And Real Gas.
From socratic.org
What is the difference between an ideal gas and a real gas? Socratic Ideal Gas And Real Gas Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic. Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible volume and that intermolecular interactions are negligible—are no longer valid. In this section, we consider the properties of real gases and how and why. Ideal Gas And Real Gas.
From www.youtube.com
Ideal vs Real Gases YouTube Ideal Gas And Real Gas We also examine liquefaction, a key property of real gases that is not. Fortunately, at the conditions of temperature and pressure that are normally. The ideal gas law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a large number of molecules, which are in random. Why do real. Ideal Gas And Real Gas.
From energiatoday.com
Ideal Gas vs Real Gas Differences and Concept Ideal Gas And Real Gas Learn the key differences between ideal gas and real gas, two types of gases that have different properties and behaviours. We also examine liquefaction, a key property of real gases that is not. Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic. Fortunately, at the conditions of temperature and pressure. Ideal Gas And Real Gas.
From byjus.com
What is ideal gas and real gas? Ideal Gas And Real Gas Why do real gases behave so differently from ideal gases at high pressures and low temperatures? Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic. Learn the key differences between ideal gas and real gas, two types of gases that have different properties and behaviours. Fortunately, at the conditions of. Ideal Gas And Real Gas.
From www.slideserve.com
PPT REAL VS IDEAL GASES PowerPoint Presentation, free download ID Ideal Gas And Real Gas We also examine liquefaction, a key property of real gases that is not. In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law. Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic. Why do real gases. Ideal Gas And Real Gas.
From www.youtube.com
The Ideal Gas Law YouTube Ideal Gas And Real Gas We also examine liquefaction, a key property of real gases that is not. Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic. In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law. Under these conditions, the. Ideal Gas And Real Gas.
From www.slideserve.com
PPT Avogadro’s Law PowerPoint Presentation, free download ID1534423 Ideal Gas And Real Gas We also examine liquefaction, a key property of real gases that is not. Why do real gases behave so differently from ideal gases at high pressures and low temperatures? In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law. Fortunately, at the conditions of temperature and. Ideal Gas And Real Gas.
From www.slideserve.com
PPT Chapter 13 Gases PowerPoint Presentation, free download ID6371960 Ideal Gas And Real Gas While the particles of an ideal gas are assumed to occupy no volume and experience no interparticle attractions, the particles of a real gas do have. Learn the key differences between ideal gas and real gas, two types of gases that have different properties and behaviours. The ideal gas law can be derived from the kinetic theory of gases and. Ideal Gas And Real Gas.
From depositphotos.com
Ideal Gas Intermolecular Forces Real Gas Attractive Forces Stock Vector Ideal Gas And Real Gas The ideal gas law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a large number of molecules, which are in random. In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law. Fortunately, at the. Ideal Gas And Real Gas.
From www.bartleby.com
Ideal and Real Gases bartleby Ideal Gas And Real Gas Why do real gases behave so differently from ideal gases at high pressures and low temperatures? While the particles of an ideal gas are assumed to occupy no volume and experience no interparticle attractions, the particles of a real gas do have. In this section, we consider the properties of real gases and how and why they differ from the. Ideal Gas And Real Gas.
From www.slideserve.com
PPT Ch. 13 Notes Gas Laws PowerPoint Presentation, free download Ideal Gas And Real Gas Fortunately, at the conditions of temperature and pressure that are normally. While the particles of an ideal gas are assumed to occupy no volume and experience no interparticle attractions, the particles of a real gas do have. In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas. Ideal Gas And Real Gas.
From www.youtube.com
Ideal Gas Vs Real Gas YouTube Ideal Gas And Real Gas The ideal gas law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a large number of molecules, which are in random. Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible volume and that intermolecular interactions are negligible—are no longer valid.. Ideal Gas And Real Gas.
From www.expii.com
Real vs. Ideal Gases — Comparison & Importance Expii Ideal Gas And Real Gas Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic. We also examine liquefaction, a key property of real gases that is not. Fortunately, at the conditions of temperature and pressure that are normally. Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible. Ideal Gas And Real Gas.
From www.slideserve.com
PPT REAL VS IDEAL GASES PowerPoint Presentation, free download ID Ideal Gas And Real Gas Why do real gases behave so differently from ideal gases at high pressures and low temperatures? Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible volume and that intermolecular interactions are negligible—are no longer valid. In this section, we consider the properties of real gases and how and why they differ from. Ideal Gas And Real Gas.
From sciencenotes.org
Ideal Gas Law Formula and Examples Ideal Gas And Real Gas Why do real gases behave so differently from ideal gases at high pressures and low temperatures? We also examine liquefaction, a key property of real gases that is not. Fortunately, at the conditions of temperature and pressure that are normally. Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible volume and that. Ideal Gas And Real Gas.
From slidesharenow.blogspot.com
Ideal Gas Law Vs Real Gas slideshare Ideal Gas And Real Gas Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible volume and that intermolecular interactions are negligible—are no longer valid. Learn the key differences between ideal gas and real gas, two types of gases that have different properties and behaviours. In this section, we consider the properties of real gases and how and. Ideal Gas And Real Gas.
From www.youtube.com
Difference between Ideal and Real Gas YouTube Ideal Gas And Real Gas The ideal gas law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a large number of molecules, which are in random. Fortunately, at the conditions of temperature and pressure that are normally. While the particles of an ideal gas are assumed to occupy no volume and experience no. Ideal Gas And Real Gas.
From slidetodoc.com
The differences between ideal gas and real gas Ideal Gas And Real Gas Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible volume and that intermolecular interactions are negligible—are no longer valid. While the particles of an ideal gas are assumed to occupy no volume and experience no interparticle attractions, the particles of a real gas do have. Fortunately, at the conditions of temperature and. Ideal Gas And Real Gas.
From www.britannica.com
Ideal gas Definition, Equation, Properties, & Facts Britannica Ideal Gas And Real Gas While the particles of an ideal gas are assumed to occupy no volume and experience no interparticle attractions, the particles of a real gas do have. In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law. Under these conditions, the two basic assumptions behind the ideal. Ideal Gas And Real Gas.
From www.slideshare.net
Ideal gas vs real gas Ideal Gas And Real Gas The ideal gas law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a large number of molecules, which are in random. Fortunately, at the conditions of temperature and pressure that are normally. Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have. Ideal Gas And Real Gas.
From www.youtube.com
Difference Between Real Gases and Ideal Gases YouTube Ideal Gas And Real Gas Fortunately, at the conditions of temperature and pressure that are normally. In this section, we consider the properties of real gases and how and why they differ from the predictions of the ideal gas law. Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible volume and that intermolecular interactions are negligible—are no. Ideal Gas And Real Gas.
From www.geeksforgeeks.org
Difference Between Ideal Gas And Real Gas Ideal Gas And Real Gas Learn the key differences between ideal gas and real gas, two types of gases that have different properties and behaviours. Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic. Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible volume and that intermolecular. Ideal Gas And Real Gas.
From pediaa.com
Difference Between Real and Ideal Gas Definition, Specific Properties Ideal Gas And Real Gas Fortunately, at the conditions of temperature and pressure that are normally. We also examine liquefaction, a key property of real gases that is not. Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible volume and that intermolecular interactions are negligible—are no longer valid. Real gases do not obey the ideal gas as. Ideal Gas And Real Gas.
From www.slideshare.net
Gas laws glk xi_chemistry Ideal Gas And Real Gas We also examine liquefaction, a key property of real gases that is not. The ideal gas law can be derived from the kinetic theory of gases and relies on the assumptions that (1) the gas consists of a large number of molecules, which are in random. In this section, we consider the properties of real gases and how and why. Ideal Gas And Real Gas.
From www.youtube.com
Ideal Versus Real Gases YouTube Ideal Gas And Real Gas Real gases do not obey the ideal gas as the collisions of the gas particles are not perfectly elastic. Under these conditions, the two basic assumptions behind the ideal gas law—namely, that gas molecules have negligible volume and that intermolecular interactions are negligible—are no longer valid. Learn the key differences between ideal gas and real gas, two types of gases. Ideal Gas And Real Gas.
From classnotes.org.in
Real Gases Chemistry, Class 11, States of Matter Ideal Gas And Real Gas Fortunately, at the conditions of temperature and pressure that are normally. Why do real gases behave so differently from ideal gases at high pressures and low temperatures? Learn the key differences between ideal gas and real gas, two types of gases that have different properties and behaviours. While the particles of an ideal gas are assumed to occupy no volume. Ideal Gas And Real Gas.