Aluminum Chloride Unbalanced Equation . Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. 1 atom in reagents and 1 atom. The balanced equation will appear. For each element, we check if the number of atoms is balanced on both sides of the equation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. \[ \ce{ ca5(po4)3(oh)(s) + h_3po4 (aq) + h_2o_{(l)} \rightarrow ca(h_2po_4)_2 \cdot h_2o_{(s)} }. Explain the role of the law of conservation of mass in a chemical reaction. Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: Balance a chemical equation when given the unbalanced equation. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Al (s) + o 2 (g) → al 2 o 3 (s) solution. The initial (unbalanced) equation is as follows: In this video we'll balance the equation alcl3 = al + cl2 and provide the correct coefficients for.
from www.numerade.com
To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Explain the role of the law of conservation of mass in a chemical reaction. Al (s) + o 2 (g) → al 2 o 3 (s) solution. Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: The balanced equation will appear. 1 atom in reagents and 1 atom. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. The initial (unbalanced) equation is as follows: Balance a chemical equation when given the unbalanced equation.
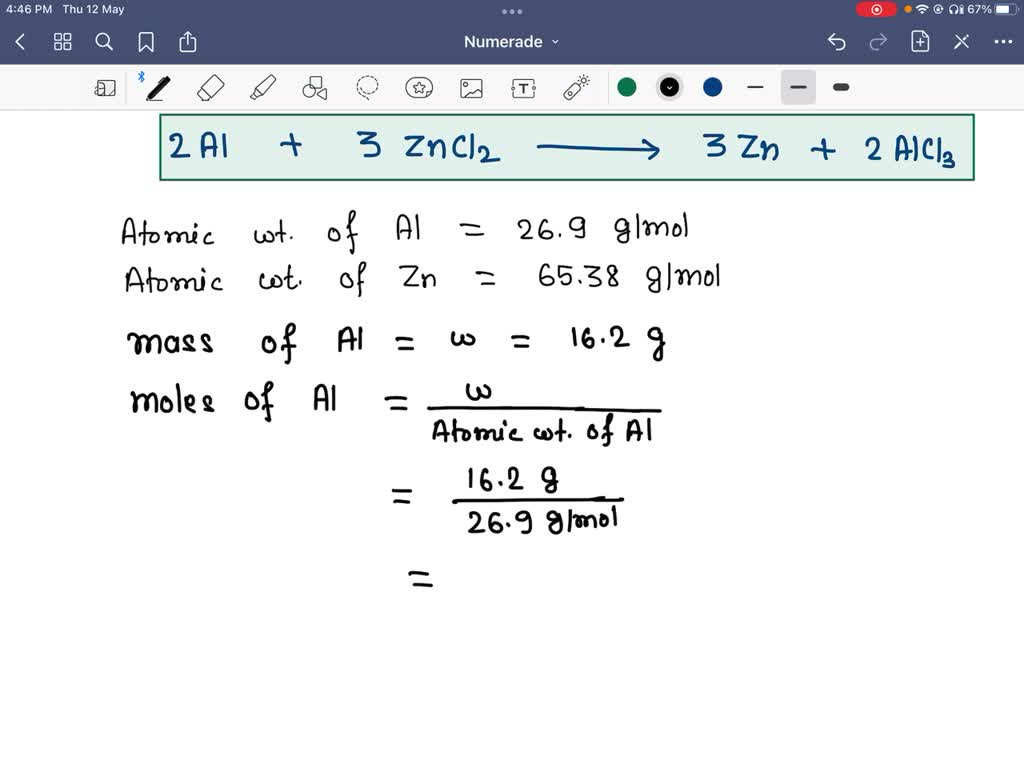
SOLVED Aluminum metal reacts with zinc chloride to produce zinc metal
Aluminum Chloride Unbalanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. In this video we'll balance the equation alcl3 = al + cl2 and provide the correct coefficients for. 1 atom in reagents and 1 atom. The initial (unbalanced) equation is as follows: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Al (s) + o 2 (g) → al 2 o 3 (s) solution. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. The balanced equation will appear. For each element, we check if the number of atoms is balanced on both sides of the equation. Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: Explain the role of the law of conservation of mass in a chemical reaction. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Balance a chemical equation when given the unbalanced equation. \[ \ce{ ca5(po4)3(oh)(s) + h_3po4 (aq) + h_2o_{(l)} \rightarrow ca(h_2po_4)_2 \cdot h_2o_{(s)} }.
From slideplayer.com
Chemical Reactions. ppt download Aluminum Chloride Unbalanced Equation The initial (unbalanced) equation is as follows: The balanced equation will appear. 1 atom in reagents and 1 atom. Balance a chemical equation when given the unbalanced equation. Explain the role of the law of conservation of mass in a chemical reaction. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via.. Aluminum Chloride Unbalanced Equation.
From slideplayer.com
Balancing Equations and Identifying Reaction Types ppt download Aluminum Chloride Unbalanced Equation Al (s) + o 2 (g) → al 2 o 3 (s) solution. Explain the role of the law of conservation of mass in a chemical reaction. \[ \ce{ ca5(po4)3(oh)(s) + h_3po4 (aq) + h_2o_{(l)} \rightarrow ca(h_2po_4)_2 \cdot h_2o_{(s)} }. The balanced equation will appear. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with. Aluminum Chloride Unbalanced Equation.
From www.numerade.com
SOLVED Aluminum metal reacts with zinc chloride to produce zinc metal Aluminum Chloride Unbalanced Equation For each element, we check if the number of atoms is balanced on both sides of the equation. The balanced equation will appear. Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: Explain the role of the law of conservation of mass in a chemical reaction. To balance a chemical equation,. Aluminum Chloride Unbalanced Equation.
From www.numerade.com
SOLVED Aluminum reacts with chlorine gas to produce aluminum chloride Aluminum Chloride Unbalanced Equation Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. \[ \ce{ ca5(po4)3(oh)(s) + h_3po4 (aq) + h_2o_{(l)} \rightarrow ca(h_2po_4)_2 \cdot h_2o_{(s)} }. For each element, we check if the number of atoms is balanced on both sides of the equation. In this video we'll. Aluminum Chloride Unbalanced Equation.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and Aluminum Chloride Unbalanced Equation In this video we'll balance the equation alcl3 = al + cl2 and provide the correct coefficients for. 1 atom in reagents and 1 atom. The initial (unbalanced) equation is as follows: The balanced equation will appear. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. For each element, we check if. Aluminum Chloride Unbalanced Equation.
From www.youtube.com
How to Draw the Lewis Structure for AlCl3 Aluminum Chloride YouTube Aluminum Chloride Unbalanced Equation For each element, we check if the number of atoms is balanced on both sides of the equation. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen,. Aluminum Chloride Unbalanced Equation.
From www.youtube.com
Aluminium reacts with chlorine gas to form aluminium chloride via the Aluminum Chloride Unbalanced Equation For each element, we check if the number of atoms is balanced on both sides of the equation. 1 atom in reagents and 1 atom. Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: Balance a chemical equation when given the unbalanced equation. Al + hcl = alcl3 + h2 is. Aluminum Chloride Unbalanced Equation.
From slideplayer.com
The Law of Conservation of Mass ppt download Aluminum Chloride Unbalanced Equation The initial (unbalanced) equation is as follows: Explain the role of the law of conservation of mass in a chemical reaction. Al (s) + o 2 (g) → al 2 o 3 (s) solution. 1 atom in reagents and 1 atom. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. In. Aluminum Chloride Unbalanced Equation.
From slidetodoc.com
Balancing Chemical Equations What is a chemical equation Aluminum Chloride Unbalanced Equation Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. \[ \ce{ ca5(po4)3(oh)(s) + h_3po4 (aq) + h_2o_{(l)} \rightarrow ca(h_2po_4)_2 \cdot h_2o_{(s)} }. Explain the role of the law of conservation of mass in a chemical reaction. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two. Aluminum Chloride Unbalanced Equation.
From slideplayer.com
Ch Chemical Reactions II. Balancing Equations. ppt download Aluminum Chloride Unbalanced Equation \[ \ce{ ca5(po4)3(oh)(s) + h_3po4 (aq) + h_2o_{(l)} \rightarrow ca(h_2po_4)_2 \cdot h_2o_{(s)} }. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. In this video we'll balance the equation alcl3 = al + cl2 and provide the correct coefficients for. For each element, we check if the number of atoms is. Aluminum Chloride Unbalanced Equation.
From www.numerade.com
SOLVED shou vour work Use the correct number of significant figures Aluminum Chloride Unbalanced Equation For each element, we check if the number of atoms is balanced on both sides of the equation. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The initial (unbalanced) equation is as follows: \[ \ce{ ca5(po4)3(oh)(s) + h_3po4 (aq) + h_2o_{(l)} \rightarrow ca(h_2po_4)_2 \cdot h_2o_{(s)} }. Balance the chemical equation for. Aluminum Chloride Unbalanced Equation.
From www.numerade.com
SOLVED Given the unbalanced equation Al + HCl > AlCl3 + H2. When a Aluminum Chloride Unbalanced Equation Al (s) + o 2 (g) → al 2 o 3 (s) solution. 1 atom in reagents and 1 atom. In this video we'll balance the equation alcl3 = al + cl2 and provide the correct coefficients for. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. \[ \ce{ ca5(po4)3(oh)(s) +. Aluminum Chloride Unbalanced Equation.
From www.numerade.com
SOLVED Write the chemical formula for aluminum chloride Answer Use Aluminum Chloride Unbalanced Equation 1 atom in reagents and 1 atom. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear. For each element, we check if the number of atoms is balanced on both sides of the equation. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction. Aluminum Chloride Unbalanced Equation.
From www.chegg.com
Solved An aqueous solution of aluminium chloride can be Aluminum Chloride Unbalanced Equation The balanced equation will appear. Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. 1 atom in reagents and 1 atom. Al + hcl = alcl3 + h2 is a single displacement (substitution). Aluminum Chloride Unbalanced Equation.
From www.numerade.com
SOLVEDZOOM + > Page of 2 Consider the unbalanced equation for the Aluminum Chloride Unbalanced Equation 1 atom in reagents and 1 atom. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Al (s) + o 2 (g) → al 2 o 3 (s) solution. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six. Aluminum Chloride Unbalanced Equation.
From www.numerade.com
SOLVED Question 11 (1 point) What is the correct unbalanced skeleton Aluminum Chloride Unbalanced Equation Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. \[ \ce{ ca5(po4)3(oh)(s) + h_3po4 (aq) + h_2o_{(l)} \rightarrow ca(h_2po_4)_2 \cdot h_2o_{(s)} }. The initial (unbalanced) equation is as follows: To balance a chemical equation, enter an equation of a chemical reaction and press the. Aluminum Chloride Unbalanced Equation.
From www.youtube.com
Q5 Write a balanced chemical equation for aluminium burning in chlorine Aluminum Chloride Unbalanced Equation In this video we'll balance the equation alcl3 = al + cl2 and provide the correct coefficients for. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. Al (s) + o 2 (g) → al 2 o 3 (s) solution. Explain the role of. Aluminum Chloride Unbalanced Equation.
From slideplayer.com
Active Learning Exercises ppt download Aluminum Chloride Unbalanced Equation The initial (unbalanced) equation is as follows: Balance a chemical equation when given the unbalanced equation. 1 atom in reagents and 1 atom. The balanced equation will appear. Al (s) + o 2 (g) → al 2 o 3 (s) solution. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Al. Aluminum Chloride Unbalanced Equation.
From stock.adobe.com
Stylized molecule model/structural formula of aluminum chloride. Stock Aluminum Chloride Unbalanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: Balance a chemical equation when given the unbalanced equation. Explain the role of the law of conservation of mass in a chemical reaction. \[ \ce{. Aluminum Chloride Unbalanced Equation.
From www.youtube.com
How to Balance AlCl3 = Al + Cl2 (Aluminum chloride by Aluminum Chloride Unbalanced Equation Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. 1 atom in reagents and 1 atom. Al (s) + o 2 (g) → al 2 o 3 (s) solution. To balance a chemical equation, enter an equation of a chemical reaction and press the. Aluminum Chloride Unbalanced Equation.
From www.numerade.com
SOLVED In the following reaction, what is the correct coefficient for Aluminum Chloride Unbalanced Equation For each element, we check if the number of atoms is balanced on both sides of the equation. The initial (unbalanced) equation is as follows: Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: The balanced equation will appear. \[ \ce{ ca5(po4)3(oh)(s) + h_3po4 (aq) + h_2o_{(l)} \rightarrow ca(h_2po_4)_2 \cdot h_2o_{(s)}. Aluminum Chloride Unbalanced Equation.
From www.youtube.com
How to write the formula for aluminum chloride YouTube Aluminum Chloride Unbalanced Equation \[ \ce{ ca5(po4)3(oh)(s) + h_3po4 (aq) + h_2o_{(l)} \rightarrow ca(h_2po_4)_2 \cdot h_2o_{(s)} }. The initial (unbalanced) equation is as follows: Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: To balance a chemical. Aluminum Chloride Unbalanced Equation.
From www.numerade.com
SOLVED Part 2 Given the following unbalanced equation KCIOs KCI + 02 Aluminum Chloride Unbalanced Equation The initial (unbalanced) equation is as follows: Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Balance a chemical equation when given the unbalanced equation. Al (s) + o 2 (g) → al 2 o 3 (s) solution. For each element, we check if the number of atoms is balanced on. Aluminum Chloride Unbalanced Equation.
From www.youtube.com
How to Write the Formula for Aluminum chloride (AlCl3) YouTube Aluminum Chloride Unbalanced Equation Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. Al (s) + o 2 (g) → al 2 o 3 (s) solution. The initial (unbalanced) equation is as follows: The balanced equation will appear. In this video we'll balance the equation alcl3 = al. Aluminum Chloride Unbalanced Equation.
From www.adda247.com
Aluminium Chloride FormulaDefinition, Structure, Uses, Properties Aluminum Chloride Unbalanced Equation The initial (unbalanced) equation is as follows: \[ \ce{ ca5(po4)3(oh)(s) + h_3po4 (aq) + h_2o_{(l)} \rightarrow ca(h_2po_4)_2 \cdot h_2o_{(s)} }. 1 atom in reagents and 1 atom. The balanced equation will appear. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Al (s) + o 2 (g) → al 2 o 3. Aluminum Chloride Unbalanced Equation.
From www.gauthmath.com
Solved Write the unbalanced chemical equation for aluminum plus Aluminum Chloride Unbalanced Equation In this video we'll balance the equation alcl3 = al + cl2 and provide the correct coefficients for. Explain the role of the law of conservation of mass in a chemical reaction. Al (s) + o 2 (g) → al 2 o 3 (s) solution. The balanced equation will appear. Al + hcl = alcl3 + h2 is a single. Aluminum Chloride Unbalanced Equation.
From slideplayer.com
Balancing Word Equations ppt download Aluminum Chloride Unbalanced Equation Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Al (s) + o 2 (g) → al 2 o 3 (s) solution. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Balance a chemical equation when given the unbalanced equation. For each element,. Aluminum Chloride Unbalanced Equation.
From www.toppr.com
How will you obtainAnhydrous aluminium chloride from alumina. Aluminum Chloride Unbalanced Equation Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium [al] and six moles of aqueous. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. For each element, we check if the number of atoms is balanced on both sides of the equation.. Aluminum Chloride Unbalanced Equation.
From www.numerade.com
SOLVED Write the equation for the of aluminum chloride Aluminum Chloride Unbalanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Al (s) + o 2 (g) → al 2 o 3 (s) solution. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. Explain the role of the law of conservation of mass in a. Aluminum Chloride Unbalanced Equation.
From www.youtube.com
Easy tips to balance Aluminium+ copper chloride= Aluminium chloride Aluminum Chloride Unbalanced Equation Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. The initial (unbalanced) equation is as follows: For each element, we check if the number of atoms is balanced on both sides of the equation. 1 atom in reagents and 1 atom. In this video we'll balance the equation alcl3 = al. Aluminum Chloride Unbalanced Equation.
From www.numerade.com
SOLVED Aluminum meta eacts with aqueous iron (II) chloride to form Aluminum Chloride Unbalanced Equation The balanced equation will appear. In this video we'll balance the equation alcl3 = al + cl2 and provide the correct coefficients for. For each element, we check if the number of atoms is balanced on both sides of the equation. Al + hcl = alcl3 + h2 is a single displacement (substitution) reaction where two moles of solid aluminium. Aluminum Chloride Unbalanced Equation.
From www.numerade.com
SOLVED Aluminium metal is added to a solution of Cu(II) ions Aluminum Chloride Unbalanced Equation \[ \ce{ ca5(po4)3(oh)(s) + h_3po4 (aq) + h_2o_{(l)} \rightarrow ca(h_2po_4)_2 \cdot h_2o_{(s)} }. The initial (unbalanced) equation is as follows: Balance a chemical equation when given the unbalanced equation. Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: In this video we'll balance the equation alcl3 = al + cl2 and. Aluminum Chloride Unbalanced Equation.
From www.numerade.com
SOLVED Aluminum metal reacts with zinc chloride to produce zinc metal Aluminum Chloride Unbalanced Equation Explain the role of the law of conservation of mass in a chemical reaction. \[ \ce{ ca5(po4)3(oh)(s) + h_3po4 (aq) + h_2o_{(l)} \rightarrow ca(h_2po_4)_2 \cdot h_2o_{(s)} }. In this video we'll balance the equation alcl3 = al + cl2 and provide the correct coefficients for. For each element, we check if the number of atoms is balanced on both sides. Aluminum Chloride Unbalanced Equation.
From slideplayer.com
Chapter 13 Review Problems ppt download Aluminum Chloride Unbalanced Equation Al (s) + o 2 (g) → al 2 o 3 (s) solution. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via. The initial (unbalanced) equation is as follows: The balanced equation will appear. For each element, we check if the number of atoms is balanced on both sides of the. Aluminum Chloride Unbalanced Equation.
From www.numerade.com
SOLVED In the following reaction, what is the correct coefficient for Aluminum Chloride Unbalanced Equation \[ \ce{ ca5(po4)3(oh)(s) + h_3po4 (aq) + h_2o_{(l)} \rightarrow ca(h_2po_4)_2 \cdot h_2o_{(s)} }. Balance the chemical equation for the reaction of aluminum metal with oxygen gas to produce solid aluminum oxide: Al (s) + o 2 (g) → al 2 o 3 (s) solution. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic. Aluminum Chloride Unbalanced Equation.