Base Definition Chemistry Quizlet . Learn about the properties of bases and see examples of bases and their uses. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Understanding acids and bases is important in chemistry. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Here's an introduction to acids and bases, with definitions for key acid and base terms. When naoh is added to water, it dissociates into component ions. How does one define acids and bases? Study with quizlet and memorize flashcards containing terms like 1 kilo (k), 1 hecto (h), 1 deka and more. The earliest definition of acids and bases is arrhenius's definition which states that: Under the arrhenius definition, acids and. In chemistry, acids and bases have been defined differently by three sets of theories.
from www.teachoo.com
Learn about the properties of bases and see examples of bases and their uses. Study with quizlet and memorize flashcards containing terms like 1 kilo (k), 1 hecto (h), 1 deka and more. Understanding acids and bases is important in chemistry. The earliest definition of acids and bases is arrhenius's definition which states that: How does one define acids and bases? In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Under the arrhenius definition, acids and. Here's an introduction to acids and bases, with definitions for key acid and base terms. When naoh is added to water, it dissociates into component ions.
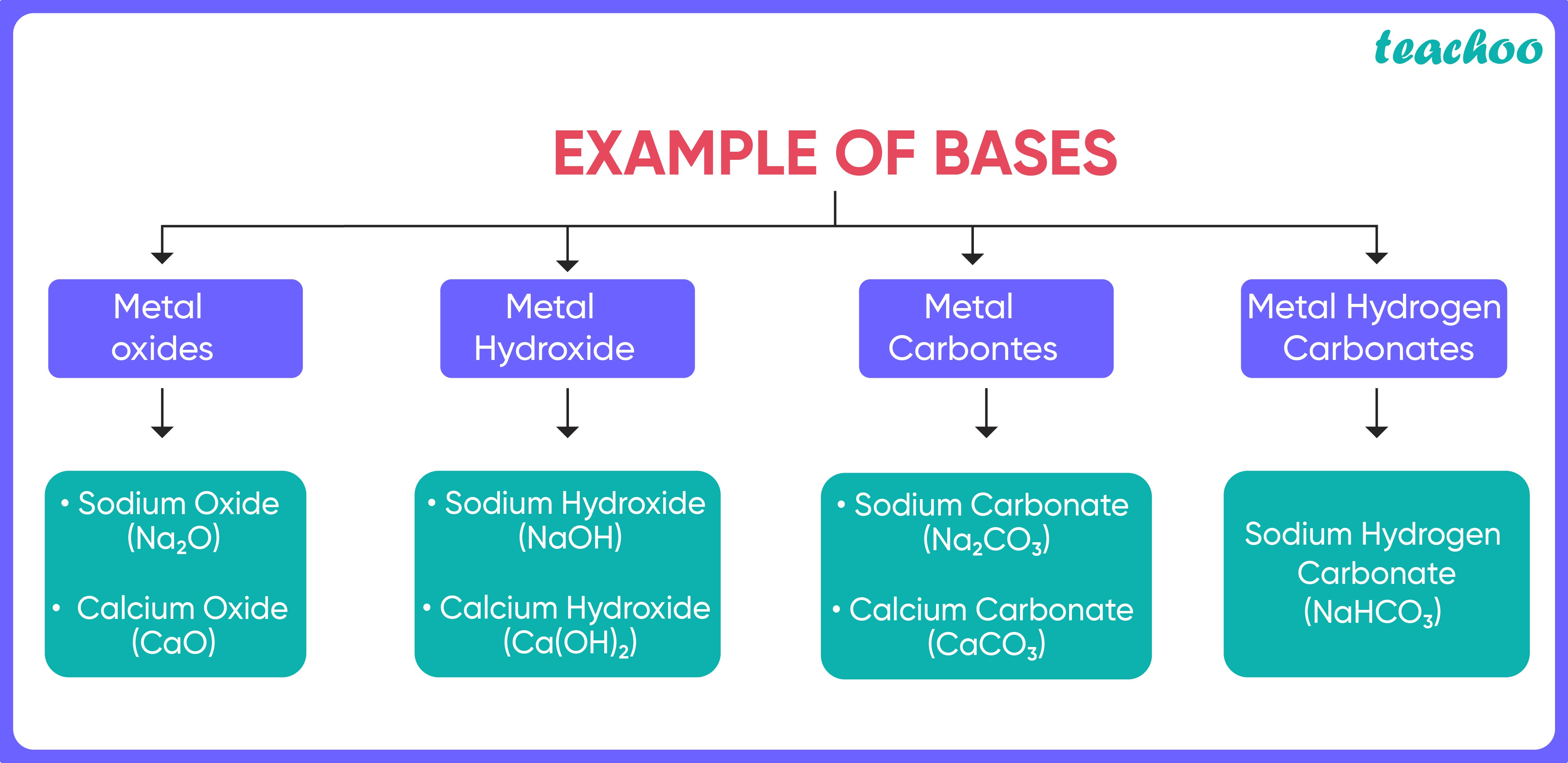
Bases and it's Properties (with Examples, Definition) Teachoo
Base Definition Chemistry Quizlet Understanding acids and bases is important in chemistry. How does one define acids and bases? Here's an introduction to acids and bases, with definitions for key acid and base terms. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. When naoh is added to water, it dissociates into component ions. Learn about the properties of bases and see examples of bases and their uses. Study with quizlet and memorize flashcards containing terms like 1 kilo (k), 1 hecto (h), 1 deka and more. Under the arrhenius definition, acids and. The earliest definition of acids and bases is arrhenius's definition which states that: An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Understanding acids and bases is important in chemistry. In chemistry, acids and bases have been defined differently by three sets of theories.
From learningillyrio1p.z14.web.core.windows.net
How To Identify Acids And Bases In A Equation Base Definition Chemistry Quizlet When naoh is added to water, it dissociates into component ions. Under the arrhenius definition, acids and. How does one define acids and bases? Learn about the properties of bases and see examples of bases and their uses. Study with quizlet and memorize flashcards containing terms like 1 kilo (k), 1 hecto (h), 1 deka and more. In chemistry, acids. Base Definition Chemistry Quizlet.
From sciencenotes.org
What Is a Base in Chemistry? Definition and Examples Base Definition Chemistry Quizlet In chemistry, acids and bases have been defined differently by three sets of theories. How does one define acids and bases? Here's an introduction to acids and bases, with definitions for key acid and base terms. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates. Base Definition Chemistry Quizlet.
From www.slideserve.com
PPT Definitions of Acids and Bases PowerPoint Presentation, free Base Definition Chemistry Quizlet Under the arrhenius definition, acids and. The earliest definition of acids and bases is arrhenius's definition which states that: Study with quizlet and memorize flashcards containing terms like 1 kilo (k), 1 hecto (h), 1 deka and more. When naoh is added to water, it dissociates into component ions. Learn about the properties of bases and see examples of bases. Base Definition Chemistry Quizlet.
From www.youtube.com
Chemistry Quizlet (States of Matter & Pure and Impure Substances) YouTube Base Definition Chemistry Quizlet An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Here's an introduction to acids and bases, with definitions for key acid and base terms. In chemistry, acids and bases have been defined differently by three sets of theories. Understanding acids and bases is important in chemistry. Learn about the. Base Definition Chemistry Quizlet.
From leah4sci.com
Definitions of Arrhenius, BronstedLowry, and Lewis Acids and Bases in Base Definition Chemistry Quizlet Here's an introduction to acids and bases, with definitions for key acid and base terms. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Understanding acids and bases is important in chemistry. Study with quizlet and memorize flashcards containing terms like 1 kilo (k), 1 hecto (h), 1 deka. Base Definition Chemistry Quizlet.
From www.slideserve.com
PPT Chapter 23 Acids, Bases, and Salts PowerPoint Presentation, free Base Definition Chemistry Quizlet In chemistry, acids and bases have been defined differently by three sets of theories. Under the arrhenius definition, acids and. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Study with quizlet and memorize flashcards containing terms like 1 kilo (k),. Base Definition Chemistry Quizlet.
From quizlet.com
unit 4, describing substances quiz 1 (elements, compounds, mixtures Base Definition Chemistry Quizlet The earliest definition of acids and bases is arrhenius's definition which states that: Under the arrhenius definition, acids and. In chemistry, acids and bases have been defined differently by three sets of theories. How does one define acids and bases? Study with quizlet and memorize flashcards containing terms like 1 kilo (k), 1 hecto (h), 1 deka and more. Here's. Base Definition Chemistry Quizlet.
From ar.inspiredpencil.com
Base Definition Chemistry Base Definition Chemistry Quizlet When naoh is added to water, it dissociates into component ions. Study with quizlet and memorize flashcards containing terms like 1 kilo (k), 1 hecto (h), 1 deka and more. The earliest definition of acids and bases is arrhenius's definition which states that: Learn about the properties of bases and see examples of bases and their uses. How does one. Base Definition Chemistry Quizlet.
From www.thoughtco.com
Base Definition in Chemistry Base Definition Chemistry Quizlet The earliest definition of acids and bases is arrhenius's definition which states that: Here's an introduction to acids and bases, with definitions for key acid and base terms. Under the arrhenius definition, acids and. Understanding acids and bases is important in chemistry. How does one define acids and bases? An acid is a substance that forms hydrogen ions h +. Base Definition Chemistry Quizlet.
From definitionklw.blogspot.com
Definition Of Base In Science DEFINITION KLW Base Definition Chemistry Quizlet Study with quizlet and memorize flashcards containing terms like 1 kilo (k), 1 hecto (h), 1 deka and more. Understanding acids and bases is important in chemistry. How does one define acids and bases? In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in. Base Definition Chemistry Quizlet.
From www.chemistrysteps.com
Organic Acids and Bases Chemistry Steps Base Definition Chemistry Quizlet Under the arrhenius definition, acids and. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. In chemistry, acids and bases have been defined differently by three sets of theories. Learn about the properties of bases and see examples of bases and. Base Definition Chemistry Quizlet.
From spmchemistry.blog.onlinetuition.com.my
Bases SPM Chemistry Base Definition Chemistry Quizlet Understanding acids and bases is important in chemistry. When naoh is added to water, it dissociates into component ions. The earliest definition of acids and bases is arrhenius's definition which states that: In chemistry, acids and bases have been defined differently by three sets of theories. An acid is a substance that forms hydrogen ions h + when dissolved in. Base Definition Chemistry Quizlet.
From elchoroukhost.net
Periodic Table Chemistry Quizlet Elcho Table Base Definition Chemistry Quizlet Study with quizlet and memorize flashcards containing terms like 1 kilo (k), 1 hecto (h), 1 deka and more. Understanding acids and bases is important in chemistry. How does one define acids and bases? In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in. Base Definition Chemistry Quizlet.
From quizlet.com
Organic Chemistry Functional Groups Diagram Quizlet Base Definition Chemistry Quizlet Here's an introduction to acids and bases, with definitions for key acid and base terms. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons. Base Definition Chemistry Quizlet.
From quizlet.com
Chemistry PreIP Intro to the Atom and Nuclear Reactions Diagram Base Definition Chemistry Quizlet Understanding acids and bases is important in chemistry. Learn about the properties of bases and see examples of bases and their uses. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Here's an introduction to acids and bases, with definitions for. Base Definition Chemistry Quizlet.
From www.geeksforgeeks.org
What are Bases? Definition, Examples, Types, Properties and Uses Base Definition Chemistry Quizlet The earliest definition of acids and bases is arrhenius's definition which states that: Learn about the properties of bases and see examples of bases and their uses. How does one define acids and bases? In chemistry, acids and bases have been defined differently by three sets of theories. An acid is a substance that forms hydrogen ions h + when. Base Definition Chemistry Quizlet.
From www.thoughtco.com
Strong Base Definition Chemistry Glossary Base Definition Chemistry Quizlet Here's an introduction to acids and bases, with definitions for key acid and base terms. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Learn about the properties of bases and see examples of bases and their uses. How does one. Base Definition Chemistry Quizlet.
From www.teachoo.com
List of Strong Bases 7+ Examples Chemistry Teachoo Base Definition Chemistry Quizlet An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Understanding acids and bases is important in chemistry. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Study with quizlet and. Base Definition Chemistry Quizlet.
From joyrtbyrd.blogspot.com
Base Meaning in Chemistry JoyrtByrd Base Definition Chemistry Quizlet Under the arrhenius definition, acids and. Here's an introduction to acids and bases, with definitions for key acid and base terms. The earliest definition of acids and bases is arrhenius's definition which states that: Learn about the properties of bases and see examples of bases and their uses. In chemistry, a base is a substance that reacts with acids to. Base Definition Chemistry Quizlet.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry Base Definition Chemistry Quizlet An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Understanding acids and bases is important in chemistry. When naoh is added to water, it dissociates into component ions. Here's an introduction to acids and bases, with definitions for key acid and base terms. In chemistry, acids and bases have. Base Definition Chemistry Quizlet.
From www.slideserve.com
PPT Acids and Bases (3) PowerPoint Presentation, free download ID Base Definition Chemistry Quizlet Here's an introduction to acids and bases, with definitions for key acid and base terms. In chemistry, acids and bases have been defined differently by three sets of theories. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. In chemistry, a base is a substance that reacts with acids. Base Definition Chemistry Quizlet.
From www.organicchemistrytutor.com
BronstedLowry AcidBase Equilibrium — Organic Chemistry Tutor Base Definition Chemistry Quizlet Study with quizlet and memorize flashcards containing terms like 1 kilo (k), 1 hecto (h), 1 deka and more. How does one define acids and bases? In chemistry, acids and bases have been defined differently by three sets of theories. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a.. Base Definition Chemistry Quizlet.
From education-portal.com
Base in Chemistry Definition & Example Video & Lesson Transcript Base Definition Chemistry Quizlet Learn about the properties of bases and see examples of bases and their uses. When naoh is added to water, it dissociates into component ions. Understanding acids and bases is important in chemistry. Here's an introduction to acids and bases, with definitions for key acid and base terms. In chemistry, acids and bases have been defined differently by three sets. Base Definition Chemistry Quizlet.
From sciencenotes.org
Arrhenius Acids and Bases Base Definition Chemistry Quizlet In chemistry, acids and bases have been defined differently by three sets of theories. The earliest definition of acids and bases is arrhenius's definition which states that: How does one define acids and bases? Under the arrhenius definition, acids and. Study with quizlet and memorize flashcards containing terms like 1 kilo (k), 1 hecto (h), 1 deka and more. Here's. Base Definition Chemistry Quizlet.
From ar.inspiredpencil.com
Base Definition Chemistry Base Definition Chemistry Quizlet Learn about the properties of bases and see examples of bases and their uses. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base. Base Definition Chemistry Quizlet.
From www.worksheetsplanet.com
Acid and Bases Differences Base Definition Chemistry Quizlet In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. When naoh is added to water, it dissociates into component ions. In chemistry, acids and bases have been defined differently by three sets of theories. How does one define acids and bases?. Base Definition Chemistry Quizlet.
From www.teachoo.com
Bases and it's Properties (with Examples, Definition) Teachoo Base Definition Chemistry Quizlet An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. In chemistry, acids and bases have been defined differently by three sets of theories. The earliest definition of acids and bases is arrhenius's definition which states that: In chemistry, a base is a substance that reacts with acids to form. Base Definition Chemistry Quizlet.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free Base Definition Chemistry Quizlet When naoh is added to water, it dissociates into component ions. An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Study. Base Definition Chemistry Quizlet.
From www.blendspace.com
Acids And Bases Introduction Lessons Blendspace Base Definition Chemistry Quizlet How does one define acids and bases? Understanding acids and bases is important in chemistry. Learn about the properties of bases and see examples of bases and their uses. In chemistry, acids and bases have been defined differently by three sets of theories. In chemistry, a base is a substance that reacts with acids to form a salt and which. Base Definition Chemistry Quizlet.
From quizlet.com
Acids and Bases, pKa Table Diagram Quizlet Base Definition Chemistry Quizlet In chemistry, acids and bases have been defined differently by three sets of theories. Here's an introduction to acids and bases, with definitions for key acid and base terms. Study with quizlet and memorize flashcards containing terms like 1 kilo (k), 1 hecto (h), 1 deka and more. Learn about the properties of bases and see examples of bases and. Base Definition Chemistry Quizlet.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID1383826 Base Definition Chemistry Quizlet How does one define acids and bases? Understanding acids and bases is important in chemistry. The earliest definition of acids and bases is arrhenius's definition which states that: In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Study with quizlet and. Base Definition Chemistry Quizlet.
From www.chemicals.co.uk
What Is a Base in Chemistry? The Chemistry Blog Base Definition Chemistry Quizlet When naoh is added to water, it dissociates into component ions. Understanding acids and bases is important in chemistry. Here's an introduction to acids and bases, with definitions for key acid and base terms. The earliest definition of acids and bases is arrhenius's definition which states that: How does one define acids and bases? Under the arrhenius definition, acids and.. Base Definition Chemistry Quizlet.
From www.hotelsrate.org
Chemical Equation Definition Chemistry Quizlet Modern Home Designs Base Definition Chemistry Quizlet In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. How does one define acids and bases? In chemistry, acids and bases have been defined differently by three sets of theories. Understanding acids and bases is important in chemistry. Here's an introduction. Base Definition Chemistry Quizlet.
From www.worksheetsplanet.com
What is a Base Definition of Base Base Definition Chemistry Quizlet How does one define acids and bases? In chemistry, a base is a substance that reacts with acids to form a salt and which releases hydroxide ions, accepts protons, or donates electrons in aqueous solution. Under the arrhenius definition, acids and. In chemistry, acids and bases have been defined differently by three sets of theories. Understanding acids and bases is. Base Definition Chemistry Quizlet.
From www.sliderbase.com
Acid/Base definitions Base Definition Chemistry Quizlet An acid is a substance that forms hydrogen ions h + when dissolved in water, and a base is a. Study with quizlet and memorize flashcards containing terms like 1 kilo (k), 1 hecto (h), 1 deka and more. When naoh is added to water, it dissociates into component ions. The earliest definition of acids and bases is arrhenius's definition. Base Definition Chemistry Quizlet.