Mhra Medical Device Class I . You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. Topical skin coupling gel for use in ultrasound scanning procedures. Building a medical device qms. Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a).
from www.ce-marking.com
We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a). You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. Building a medical device qms. This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. Topical skin coupling gel for use in ultrasound scanning procedures.
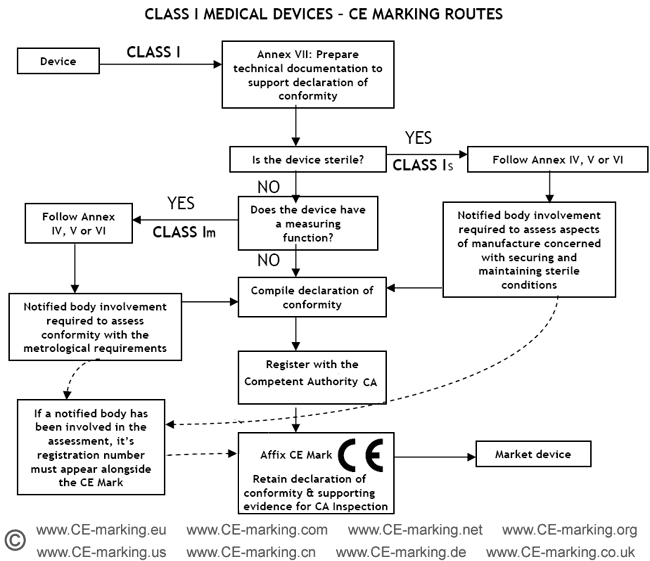
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark
Mhra Medical Device Class I Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a). Building a medical device qms. A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. Topical skin coupling gel for use in ultrasound scanning procedures.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Mhra Medical Device Class I We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a). Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. Building a medical device qms. A statutory instrument (si) laid in parliament today, 21 october, will provide a new. Mhra Medical Device Class I.
From gbu-taganskij.ru
Medical Device Classification According To The MDR Complete, 60 OFF Mhra Medical Device Class I You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. Topical skin coupling gel for use in ultrasound scanning procedures. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. A statutory instrument (si) laid in parliament today,. Mhra Medical Device Class I.
From www.scribd.com
MHRA Medical Devices PDF PDF Clinical Trial Medical Device Mhra Medical Device Class I Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. Topical skin coupling gel for use in ultrasound scanning procedures. We have prepared guidance on registration of certain medical devices which are. Mhra Medical Device Class I.
From international.cliniexperts.com
How to register medical devices and IVDs in the UK CliniExperts Mhra Medical Device Class I This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a). A statutory instrument (si) laid in. Mhra Medical Device Class I.
From www.linkedin.com
MHRA publish an update on future medical device regulations Mhra Medical Device Class I You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach. Mhra Medical Device Class I.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Mhra Medical Device Class I We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a). You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices.. Mhra Medical Device Class I.
From learn.marsdd.com
Medical device regulations, classification & submissions Canada, US, EU Mhra Medical Device Class I Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a). Building. Mhra Medical Device Class I.
From www.regdesk.co
MHRA Guidance on Registration of Medical Devices RegDesk Mhra Medical Device Class I Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a). Topical skin coupling gel for use in ultrasound scanning procedures. You can place a class i medical device which. Mhra Medical Device Class I.
From operonstrategist.com
A Comprehensive Guide to MHRA Medical Device Registration (Steps Mhra Medical Device Class I This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. You can place a class i. Mhra Medical Device Class I.
From www.i3cglobal.com
UKCA Process Chart for Class 1 Device Self Declaration Mhra Medical Device Class I Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework. Mhra Medical Device Class I.
From www.pixformance.com
Physiotherapy Pixformance Sports GmbH Mhra Medical Device Class I We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a). Building a medical device qms. You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. The adoption of a risk stratification approach for ivds, adopting. Mhra Medical Device Class I.
From operonstrategist.com
A Comprehensive Guide to MHRA Medical Device Registration (Steps Mhra Medical Device Class I A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. The adoption of a risk stratification. Mhra Medical Device Class I.
From www.lexology.com
The MHRA's recent updates to the regulation of medical devices Lexology Mhra Medical Device Class I You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. Building a medical device qms. Topical skin coupling gel for use in ultrasound scanning procedures. A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. This guidance. Mhra Medical Device Class I.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Mhra Medical Device Class I A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a). Building a medical device qms.. Mhra Medical Device Class I.
From www.gs1uk.org
GS1 UK What you need to know about the MHRA consultation on medical Mhra Medical Device Class I A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. Topical skin coupling gel for use in ultrasound scanning procedures. Building a medical device qms. Current manifestations of ai in veterinary medicine are. Mhra Medical Device Class I.
From gbu-taganskij.ru
MHRA's Guide To The New EU Medical Devices Regulations, 43 OFF Mhra Medical Device Class I Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. You can place a class i medical. Mhra Medical Device Class I.
From www.vrogue.co
Get Mhra Registration For Ukca Certification For Medi vrogue.co Mhra Medical Device Class I We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a). This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. The adoption of a. Mhra Medical Device Class I.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Mhra Medical Device Class I Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. Building a medical device qms. A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum. Mhra Medical Device Class I.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Mhra Medical Device Class I This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. Topical skin coupling gel for use in ultrasound scanning procedures. Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and. Mhra Medical Device Class I.
From cortex-design.com
Cortex Design • What's My FDA Medical Device Classification? Mhra Medical Device Class I Building a medical device qms. Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. This guidance applies to manufacturers of class i medical devices, including. Mhra Medical Device Class I.
From www.ce-marking.eu
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark Mhra Medical Device Class I This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. A statutory instrument. Mhra Medical Device Class I.
From www.youtube.com
StepbyStep Guide How to Get UK MHRA Registration for Medical Devices Mhra Medical Device Class I You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem. Mhra Medical Device Class I.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Mhra Medical Device Class I Topical skin coupling gel for use in ultrasound scanning procedures. Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a). You can place a class i medical device which. Mhra Medical Device Class I.
From www.scribd.com
Managing Medical Devices MHRA Medical Device Reliability Engineering Mhra Medical Device Class I This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. Topical skin coupling gel for use in ultrasound scanning procedures. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. You can place a class i medical device which has a sterile or measuring function with a. Mhra Medical Device Class I.
From www.ce-marking.com
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark Mhra Medical Device Class I A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a). Topical skin coupling gel for use in ultrasound scanning procedures. The adoption of a risk stratification approach for ivds, adopting. Mhra Medical Device Class I.
From www.scribd.com
Mhra (Mca and Mda) PDF Medical Device Health Care Mhra Medical Device Class I Topical skin coupling gel for use in ultrasound scanning procedures. You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. This guidance applies to manufacturers of class. Mhra Medical Device Class I.
From gbu-taganskij.ru
MHRA's Guide To The New EU Medical Devices Regulations, 43 OFF Mhra Medical Device Class I Topical skin coupling gel for use in ultrasound scanning procedures. A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. Building a medical device qms. Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. We have prepared guidance on registration. Mhra Medical Device Class I.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Mhra Medical Device Class I This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. Topical skin coupling gel for use in ultrasound scanning procedures. Building a medical device qms. Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. The adoption of a risk stratification approach for ivds, adopting. Mhra Medical Device Class I.
From spyro-soft.com
EU MDR everything you need to know about Medical Device Regulation Mhra Medical Device Class I The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. You can place a class i medical device. Mhra Medical Device Class I.
From www.arenasolutions.com
Class I Device Definition Arena Mhra Medical Device Class I This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and seem to be. Topical skin coupling gel for use in ultrasound scanning procedures. A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory. Mhra Medical Device Class I.
From www.slideserve.com
PPT MHRA Guidelines. Understanding how to improve practice/safety Mhra Medical Device Class I We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a). You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. Topical skin coupling gel for use in ultrasound scanning procedures. This guidance applies to manufacturers. Mhra Medical Device Class I.
From vem-medical.com
Medical Device Manufacturing Mhra Medical Device Class I A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. Building a medical device qms. Topical skin coupling gel for use in ultrasound scanning procedures. Current manifestations. Mhra Medical Device Class I.
From www.regdesk.co
MHRA Guidance on Medical Software and Applications RegDesk Mhra Medical Device Class I You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. We have prepared guidance on registration of certain medical devices which are eu mdd class i medical devices which are a). Topical skin coupling gel for use in ultrasound scanning procedures. The adoption of a risk. Mhra Medical Device Class I.
From www.cognidox.com
New IVD regulation is coming. are you ready? Mhra Medical Device Class I Topical skin coupling gel for use in ultrasound scanning procedures. The adoption of a risk stratification approach for ivds, adopting the international medical device regulators forum approach of. This guidance applies to manufacturers of class i medical devices, including accessories but excluding devices. Current manifestations of ai in veterinary medicine are poor examples of what this technology can be and. Mhra Medical Device Class I.
From mavink.com
Types Of Medical Devices List Mhra Medical Device Class I You can place a class i medical device which has a sterile or measuring function with a valid mdd certificate on the gb market. Topical skin coupling gel for use in ultrasound scanning procedures. A statutory instrument (si) laid in parliament today, 21 october, will provide a new regulatory framework meaning that medicines. Building a medical device qms. The adoption. Mhra Medical Device Class I.