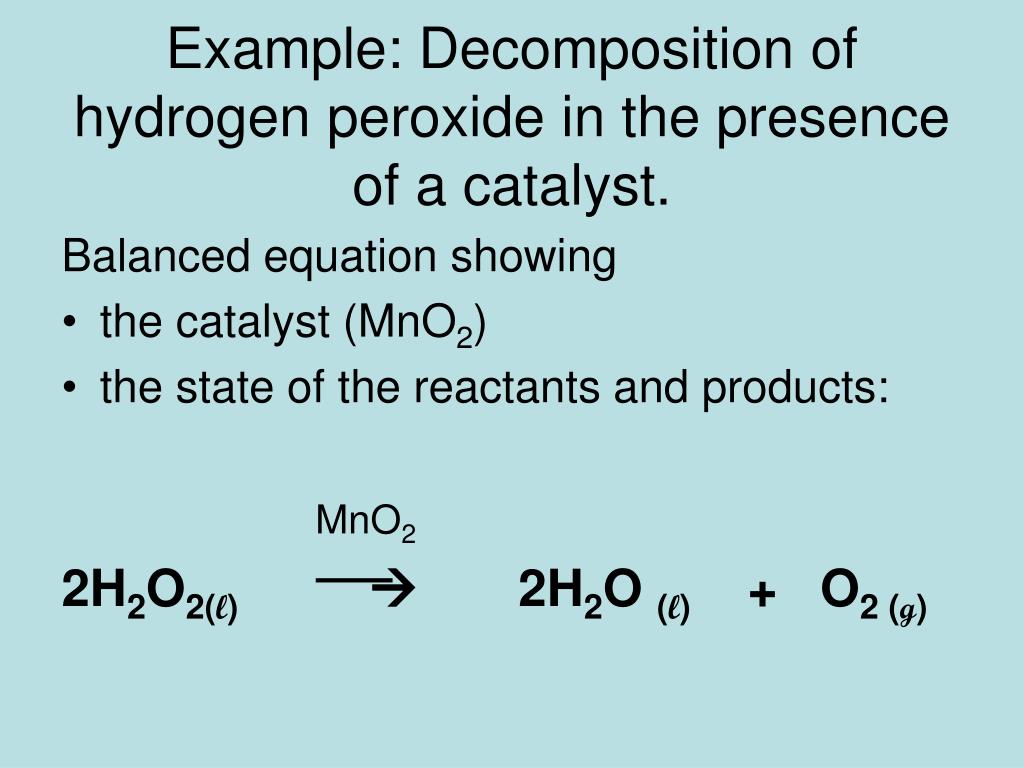
Manganese Iv Oxide Reacts With Hydrogen Peroxide . The amount of energy given off in this very. Includes kit list and safety instructions. Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Bubbles of o₂ will form immediately (the. Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. This page looks at some aspects of manganese chemistry. Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. Pour some hydrogen peroxide into the cylinder and add a small spatula scoop of mno₂. One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one mole of water [h 2 o] and one mole of manganese dioxide [mno 2] But what are the intermediates in this catalyzed reaction?
from www.tessshebaylo.com
The amount of energy given off in this very. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one mole of water [h 2 o] and one mole of manganese dioxide [mno 2] Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. This page looks at some aspects of manganese chemistry. Bubbles of o₂ will form immediately (the. But what are the intermediates in this catalyzed reaction? Pour some hydrogen peroxide into the cylinder and add a small spatula scoop of mno₂. Includes kit list and safety instructions. Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide.
Hydrogen Peroxide Manganese Dioxide Chemical Equation Tessshebaylo
Manganese Iv Oxide Reacts With Hydrogen Peroxide Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. This page looks at some aspects of manganese chemistry. Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. But what are the intermediates in this catalyzed reaction? Includes kit list and safety instructions. Pour some hydrogen peroxide into the cylinder and add a small spatula scoop of mno₂. Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one mole of water [h 2 o] and one mole of manganese dioxide [mno 2] Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The amount of energy given off in this very. Bubbles of o₂ will form immediately (the.
From www.nagwa.com
Question Video Identifying the Correct Statement For the Manganese Iv Oxide Reacts With Hydrogen Peroxide Pour some hydrogen peroxide into the cylinder and add a small spatula scoop of mno₂. This page looks at some aspects of manganese chemistry. Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. Bubbles of o₂ will form immediately (the. The amount of energy given off in this very. Manganese dioxide. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.numerade.com
SOLVED What happened when the manganese (IV) oxide, MnO2, was added to Manganese Iv Oxide Reacts With Hydrogen Peroxide Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. The amount of energy given off in this very. Pour some hydrogen peroxide into the cylinder and add a small spatula scoop of mno₂. One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one mole of water [h 2 o]. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.tessshebaylo.com
Balanced Equation For The Breakdown Of Hydrogen Peroxide By Manganese Manganese Iv Oxide Reacts With Hydrogen Peroxide The amount of energy given off in this very. This page looks at some aspects of manganese chemistry. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. Includes kit list and safety instructions. Pour some hydrogen peroxide into the. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From segudanggambar875.blogspot.com
Hydrogen Peroxide And Manganese Dioxide Balanced Equation Hydrogen Manganese Iv Oxide Reacts With Hydrogen Peroxide Includes kit list and safety instructions. Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. The amount of energy given off in this very. One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii). Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From hydrogenpotanezu.blogspot.com
Hydrogen Manganese Dioxide And Hydrogen Peroxide Manganese Iv Oxide Reacts With Hydrogen Peroxide Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. Pour some hydrogen peroxide into the cylinder and add a small spatula scoop of mno₂. The amount of energy given off in this very. Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. Includes kit list and safety instructions. One mole. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.tessshebaylo.com
Chemical Equation For The Reaction Between Hydrogen Peroxide And Manganese Iv Oxide Reacts With Hydrogen Peroxide Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. But what are the intermediates in this catalyzed reaction? Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. This page looks at some aspects of manganese chemistry. One mole of hydrogen. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.sciencephoto.com
Manganese dioxide in hydrogen peroxide. Stock Image C035/9332 Manganese Iv Oxide Reacts With Hydrogen Peroxide Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Bubbles of o₂ will form immediately (the. The amount of energy given. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.numerade.com
An aqueous solution of hydrogen peroxide very slowly at room Manganese Iv Oxide Reacts With Hydrogen Peroxide One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one mole of water [h 2 o] and one mole of manganese dioxide [mno 2] Includes kit list and safety instructions. But what are the intermediates in this catalyzed reaction? Bubbles of o₂ will form immediately (the. This page looks at. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.tessshebaylo.com
Hydrogen Peroxide Manganese Iv Oxide Balanced Equation Tessshebaylo Manganese Iv Oxide Reacts With Hydrogen Peroxide But what are the intermediates in this catalyzed reaction? Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one mole of water [h 2 o] and one mole of manganese dioxide [mno 2] Manganese dioxide acts as a catalyst to. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.youtube.com
Catalytic effect of Manganese (IV) oxide with Hydrogen Peroxide YouTube Manganese Iv Oxide Reacts With Hydrogen Peroxide Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. The amount of energy given off in this very. Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. But what are the intermediates in this catalyzed reaction? Includes kit list and safety instructions. This page looks. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.gauthmath.com
Solved 9. Oxygen is produced by the of hydrogen peroxide Manganese Iv Oxide Reacts With Hydrogen Peroxide Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. The amount of energy given off in this very. But what are the intermediates in this catalyzed reaction? Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. This page looks at some aspects of manganese chemistry. Bubbles of o₂ will form immediately (the. Includes kit list. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From 9to5science.com
[Solved] Reaction intermediates of MnO2 catalyzed H2O2 9to5Science Manganese Iv Oxide Reacts With Hydrogen Peroxide Bubbles of o₂ will form immediately (the. Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. This page looks at some aspects of manganese chemistry. But what are the intermediates in this catalyzed reaction? Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. Collect a range of catalysts to explore. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From segudanggambar875.blogspot.com
Hydrogen Peroxide And Manganese Dioxide Balanced Equation Hydrogen Manganese Iv Oxide Reacts With Hydrogen Peroxide Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. This page looks at some aspects of manganese chemistry. Bubbles of o₂ will form immediately (the. Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. But. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From segudanggambar875.blogspot.com
Hydrogen Peroxide And Manganese Dioxide Balanced Equation Hydrogen Manganese Iv Oxide Reacts With Hydrogen Peroxide Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. But what are the intermediates in this catalyzed reaction? Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. Includes kit list and safety instructions. Collect a range of. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.alamy.com
Catalysed Adding manganese (IV) oxide (MnO2) to hydrogen Manganese Iv Oxide Reacts With Hydrogen Peroxide Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. Includes kit list and safety instructions. Bubbles of o₂ will form immediately (the. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. One mole of hydrogen peroxide [h 2 o 2]. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.youtube.com
Catalytic of hydrogen peroxide using Manganese(IV)oxide Manganese Iv Oxide Reacts With Hydrogen Peroxide This page looks at some aspects of manganese chemistry. Bubbles of o₂ will form immediately (the. One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one mole of water [h 2 o] and one mole of manganese dioxide [mno 2] Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide.. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.tessshebaylo.com
Hydrogen Peroxide And Manganese Dioxide Balanced Chemical Equation Manganese Iv Oxide Reacts With Hydrogen Peroxide Pour some hydrogen peroxide into the cylinder and add a small spatula scoop of mno₂. Bubbles of o₂ will form immediately (the. Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. The amount of energy given off in this very. Includes kit list and safety instructions. Manganese dioxide catalyzes the decomposition. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.youtube.com
Hydrogen peroxide and manganese dioxide C0078 YouTube Manganese Iv Oxide Reacts With Hydrogen Peroxide Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one mole of water [h 2 o] and one mole of manganese dioxide [mno 2] The amount of energy given off in this very. Manganese dioxide catalyzes the decomposition of hydrogen. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.numerade.com
SOLVEDA common demonstration in chemistry courses involves adding a Manganese Iv Oxide Reacts With Hydrogen Peroxide Bubbles of o₂ will form immediately (the. One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one mole of water [h 2 o] and one mole of manganese dioxide [mno 2] Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. Pour some hydrogen peroxide into the cylinder and add. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.youtube.com
of hydrogen peroxide with manganese dioxide YouTube Manganese Iv Oxide Reacts With Hydrogen Peroxide Includes kit list and safety instructions. Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. Bubbles of o₂ will form immediately (the. Pour some hydrogen peroxide into the cylinder and add a small spatula scoop of mno₂. But what are the intermediates in this catalyzed reaction? The amount of energy given off in this very. Hydrogen. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.tessshebaylo.com
Hydrogen Peroxide Manganese Dioxide Chemical Equation Tessshebaylo Manganese Iv Oxide Reacts With Hydrogen Peroxide One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one mole of water [h 2 o] and one mole of manganese dioxide [mno 2] Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. Manganese dioxide catalyzes the decomposition of hydrogen peroxide. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.tessshebaylo.com
Hydrogen Peroxide Manganese Dioxide Chemical Equation Tessshebaylo Manganese Iv Oxide Reacts With Hydrogen Peroxide The amount of energy given off in this very. Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. Includes kit list. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.tessshebaylo.com
Hydrogen Peroxide Manganese Iv Oxide Balanced Equation Tessshebaylo Manganese Iv Oxide Reacts With Hydrogen Peroxide Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Bubbles of o₂ will form immediately (the. Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.numerade.com
SOLVEDA common demonstration in chemistry courses involves adding a Manganese Iv Oxide Reacts With Hydrogen Peroxide Pour some hydrogen peroxide into the cylinder and add a small spatula scoop of mno₂. Bubbles of o₂ will form immediately (the. Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. But what are the intermediates in this catalyzed reaction? Collect a range of catalysts to explore the decomposition of hydrogen. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From journals.asm.org
Manganese Oxide Biomineralization Provides Protection against Nitrite Manganese Iv Oxide Reacts With Hydrogen Peroxide Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. Bubbles of o₂ will form immediately (the. The amount of energy given off in this very. Includes kit list and safety instructions. Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. Pour some hydrogen peroxide into the cylinder and add a small spatula scoop of mno₂.. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.researchgate.net
A.) Chemical reaction between hydrogen peroxide and cerium (IV Manganese Iv Oxide Reacts With Hydrogen Peroxide Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. But what are the intermediates in this catalyzed reaction? Includes kit list and safety instructions. Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. The amount of energy given off in this very. This page looks at some aspects of manganese chemistry. Bubbles of o₂ will. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.tessshebaylo.com
Hydrogen Peroxide Manganese Dioxide Balanced Equation Tessshebaylo Manganese Iv Oxide Reacts With Hydrogen Peroxide Pour some hydrogen peroxide into the cylinder and add a small spatula scoop of mno₂. Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. Includes kit list and safety instructions. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. Hydrogen peroxide + manganese(ii) oxide =. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From segudanggambar875.blogspot.com
Hydrogen Peroxide And Manganese Dioxide Balanced Equation Hydrogen Manganese Iv Oxide Reacts With Hydrogen Peroxide This page looks at some aspects of manganese chemistry. Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. But what are the intermediates in this catalyzed reaction? The amount of energy given off in this very. Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. Manganese dioxide catalyzes the decomposition. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.tessshebaylo.com
Hydrogen Peroxide Half Equation Tessshebaylo Manganese Iv Oxide Reacts With Hydrogen Peroxide Includes kit list and safety instructions. Pour some hydrogen peroxide into the cylinder and add a small spatula scoop of mno₂. The amount of energy given off in this very. Bubbles of o₂ will form immediately (the. One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one mole of water. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.alamy.com
Full labelled diagram of the laboratory preparation of oxygen from Manganese Iv Oxide Reacts With Hydrogen Peroxide Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. Includes kit list and safety instructions. One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one mole of water [h 2 o] and one mole of manganese dioxide [mno 2] This page looks at some aspects of manganese chemistry. Pour. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.alamy.com
making oxygen through the chemical reaction between Manganese oxide and Manganese Iv Oxide Reacts With Hydrogen Peroxide Hydrogen peroxide + manganese(ii) oxide = water + manganese dioxide. Pour some hydrogen peroxide into the cylinder and add a small spatula scoop of mno₂. This page looks at some aspects of manganese chemistry. Bubbles of o₂ will form immediately (the. But what are the intermediates in this catalyzed reaction? Manganese dioxide acts as a catalyst to decompose the hydrogen. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.slideserve.com
PPT Air PowerPoint Presentation, free download ID1879186 Manganese Iv Oxide Reacts With Hydrogen Peroxide The amount of energy given off in this very. Pour some hydrogen peroxide into the cylinder and add a small spatula scoop of mno₂. But what are the intermediates in this catalyzed reaction? This page looks at some aspects of manganese chemistry. Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. One mole of hydrogen peroxide. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.tessshebaylo.com
Hydrogen Peroxide Manganese Dioxide Balanced Equation Tessshebaylo Manganese Iv Oxide Reacts With Hydrogen Peroxide Manganese dioxide catalyzes the decomposition of hydrogen peroxide to water and oxygen gas. One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one mole of water [h 2 o] and one mole of manganese dioxide [mno 2] Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.chegg.com
Solved 3. Manganate(VII) ions, MnO4, oxidise hydrogen Manganese Iv Oxide Reacts With Hydrogen Peroxide Manganese dioxide acts as a catalyst to decompose the hydrogen peroxide which releases oxygen, water, and heat energy. Collect a range of catalysts to explore the decomposition of hydrogen peroxide, paying close attention to the varied reaction rates. This page looks at some aspects of manganese chemistry. Bubbles of o₂ will form immediately (the. Manganese dioxide catalyzes the decomposition of. Manganese Iv Oxide Reacts With Hydrogen Peroxide.
From www.tessshebaylo.com
Balanced Chemical Equation For Hydrogen Peroxide And Manganese Iv Oxide Manganese Iv Oxide Reacts With Hydrogen Peroxide Bubbles of o₂ will form immediately (the. One mole of hydrogen peroxide [h 2 o 2] and one mole of manganese(ii) oxide [mno] react to form one mole of water [h 2 o] and one mole of manganese dioxide [mno 2] But what are the intermediates in this catalyzed reaction? This page looks at some aspects of manganese chemistry. Manganese. Manganese Iv Oxide Reacts With Hydrogen Peroxide.