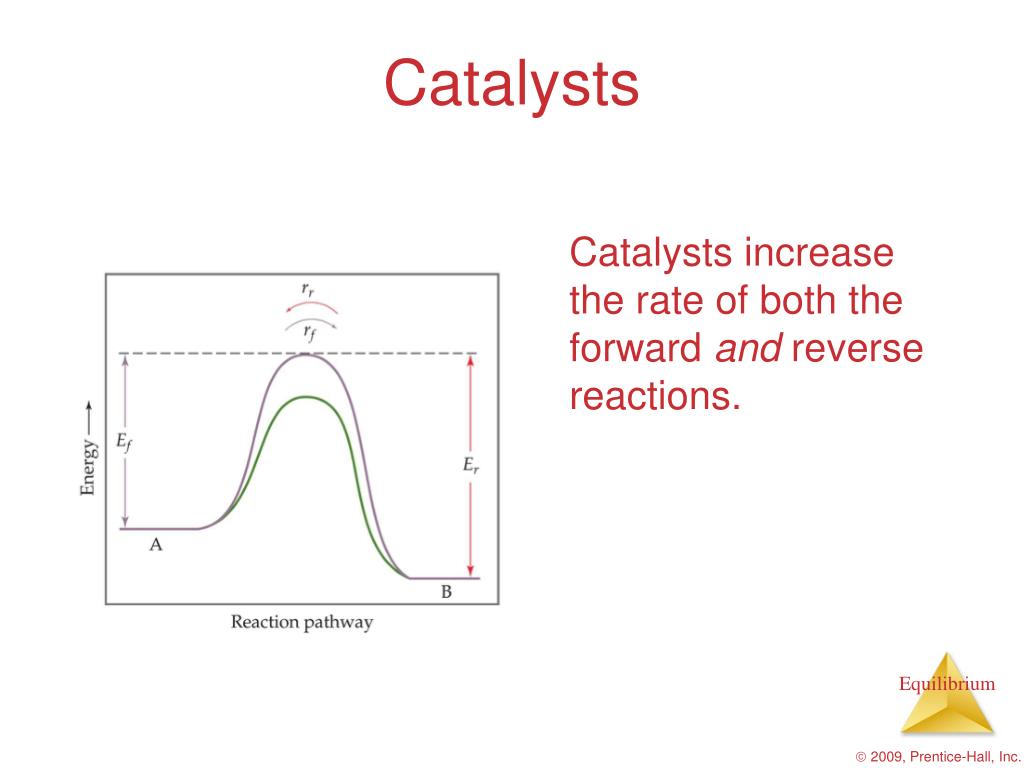
Catalyst Equilibrium Graph . The two reaction diagrams here represent the same reaction: Reaction diagrams for catalyzed reactions. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. One without a catalyst and one with a. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. It assumes that you are already familiar with. Catalysts function by providing an alternate. The only effect of the. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts function by providing an alternate. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. The effect of catalysts on reaction rates.
from www.slideserve.com
This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. It assumes that you are already familiar with. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts function by providing an alternate. One without a catalyst and one with a. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. The effect of catalysts on reaction rates. Catalysts can be homogenous (in the same phase as the reactants) or. The two reaction diagrams here represent the same reaction: Reaction diagrams for catalyzed reactions.
PPT Chapter 12 Chemical Equilibrium PowerPoint Presentation, free download ID6308959
Catalyst Equilibrium Graph Catalysts function by providing an alternate. It assumes that you are already familiar with. This page describes and explains the way that adding a catalyst affects the rate of a reaction. The effect of catalysts on reaction rates. One without a catalyst and one with a. Catalysts function by providing an alternate. The two reaction diagrams here represent the same reaction: This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. The only effect of the. Catalysts function by providing an alternate. Reaction diagrams for catalyzed reactions. Catalysts can be homogenous (in the same phase as the reactants) or.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.21C Understand Why a Catalyst Does Not Affect the Position of Catalyst Equilibrium Graph A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Reaction diagrams for catalyzed reactions. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts function by providing an. Catalyst Equilibrium Graph.
From www.vedantu.com
Le Chatelier’s Principle Laws, Principle Example and FAQs for JEE Catalyst Equilibrium Graph This page describes and explains the way that adding a catalyst affects the rate of a reaction. The effect of catalysts on reaction rates. It assumes that you are already familiar with. Reaction diagrams for catalyzed reactions. Catalysts function by providing an alternate. The only effect of the. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation.. Catalyst Equilibrium Graph.
From www.slideserve.com
PPT Rates of Reaction & Equilibrium PowerPoint Presentation, free download ID5783086 Catalyst Equilibrium Graph The effect of catalysts on reaction rates. The two reaction diagrams here represent the same reaction: This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts can be homogenous (in the same phase as the reactants) or. Reaction diagrams for catalyzed reactions. One without a catalyst and one with a. It assumes. Catalyst Equilibrium Graph.
From www.savemyexams.com
The Position of Equilibrium Edexcel IGCSE Chemistry Revision Notes 2019 Catalyst Equilibrium Graph The two reaction diagrams here represent the same reaction: Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. It assumes that you are already familiar with. Catalysts function by providing an alternate. Catalysts function by providing an alternate. This. Catalyst Equilibrium Graph.
From psu.pb.unizin.org
12.7 Catalysis Chemistry 112 Chapters 1217 of OpenStax General Chemistry Catalyst Equilibrium Graph A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. It assumes that you are already familiar with. Reaction diagrams for catalyzed reactions. Catalysts function by providing an alternate. This graph compares. Catalyst Equilibrium Graph.
From nesslabs.com
Activation energy the chemistry of getting started Ness Labs Catalyst Equilibrium Graph The two reaction diagrams here represent the same reaction: The only effect of the. Catalysts can be homogenous (in the same phase as the reactants) or. It assumes that you are already familiar with. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. This page describes and explains the. Catalyst Equilibrium Graph.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry Catalyst Equilibrium Graph The effect of catalysts on reaction rates. One without a catalyst and one with a. The only effect of the. The two reaction diagrams here represent the same reaction: This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. It assumes that you are already familiar with. Catalysts affect the rate of a chemical reaction by altering its. Catalyst Equilibrium Graph.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review PowerPoint Presentation ID6055094 Catalyst Equilibrium Graph One without a catalyst and one with a. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts function by providing an alternate. Reaction diagrams for catalyzed reactions. The only effect of the. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. The two reaction diagrams here represent the. Catalyst Equilibrium Graph.
From socratic.org
What will occur if a catalyst is added to a reaction mixture? Socratic Catalyst Equilibrium Graph This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. The effect of catalysts on reaction rates. One without a catalyst and one with a. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Catalysts affect the rate. Catalyst Equilibrium Graph.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID3480898 Catalyst Equilibrium Graph It assumes that you are already familiar with. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Reaction diagrams for catalyzed reactions. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. The two reaction diagrams here represent the same reaction: Catalysts affect the rate of a. Catalyst Equilibrium Graph.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalyst Equilibrium Graph Catalysts can be homogenous (in the same phase as the reactants) or. Reaction diagrams for catalyzed reactions. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. The two reaction diagrams here represent the same reaction: A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Catalysts affect. Catalyst Equilibrium Graph.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Catalyst Equilibrium Graph The effect of catalysts on reaction rates. One without a catalyst and one with a. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Reaction diagrams for catalyzed reactions. The only effect of the. It assumes that you are already familiar with. The two reaction diagrams here represent the. Catalyst Equilibrium Graph.
From philschatz.com
Catalysis · Chemistry Catalyst Equilibrium Graph This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. The effect of catalysts on reaction rates. The only effect of. Catalyst Equilibrium Graph.
From www.youtube.com
How to Answer Equilibrium Graph Exam Questions // HSC Chemistry YouTube Catalyst Equilibrium Graph It assumes that you are already familiar with. Catalysts function by providing an alternate. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Catalysts can be homogenous (in the same phase as the reactants) or. A catalyst speeds up. Catalyst Equilibrium Graph.
From www.catalystseurope.org
What are catalysts? Catalyst Equilibrium Graph The two reaction diagrams here represent the same reaction: One without a catalyst and one with a. Catalysts function by providing an alternate. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Catalysts function by providing an alternate. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Reaction diagrams for catalyzed reactions. Catalysts can. Catalyst Equilibrium Graph.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID3558810 Catalyst Equilibrium Graph The effect of catalysts on reaction rates. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts function by providing an alternate. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts affect the rate of a. Catalyst Equilibrium Graph.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and reversible reactions Catalyst Equilibrium Graph Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. The effect of catalysts on reaction rates. Reaction diagrams for catalyzed reactions. Catalysts function by providing an alternate. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Catalysts function by providing an alternate. It assumes that you are already. Catalyst Equilibrium Graph.
From www.slideserve.com
PPT Chapter 14 Chemical Equilibrium PowerPoint Presentation, free download ID6591070 Catalyst Equilibrium Graph A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Reaction diagrams for catalyzed reactions. Catalysts function by providing an alternate. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. One without a catalyst and one with a. This page describes and explains the way that adding. Catalyst Equilibrium Graph.
From socratic.org
What happens as reactants get consumed while approaching a chemical equilibrium? Socratic Catalyst Equilibrium Graph Catalysts can be homogenous (in the same phase as the reactants) or. One without a catalyst and one with a. This page describes and explains the way that adding a catalyst affects the rate of a reaction. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. Catalysts function by providing an. Catalyst Equilibrium Graph.
From www.researchgate.net
Equilibrium catalyst properties. Download Scientific Diagram Catalyst Equilibrium Graph The effect of catalysts on reaction rates. Reaction diagrams for catalyzed reactions. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Catalysts function by providing an alternate. Catalysts function by providing an alternate. The only effect of the. This page describes and explains the way that adding a catalyst affects the rate of a reaction. A catalyst. Catalyst Equilibrium Graph.
From www.waca.msf.org
Catalyst, Catalyst Catalyst Equilibrium Graph One without a catalyst and one with a. The effect of catalysts on reaction rates. It assumes that you are already familiar with. Catalysts function by providing an alternate. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Reaction diagrams for catalyzed reactions. This graph compares the reaction coordinates. Catalyst Equilibrium Graph.
From architecturalstudio.com
Energy Profile Diagram Catalyst Equilibrium Graph The effect of catalysts on reaction rates. It assumes that you are already familiar with. Reaction diagrams for catalyzed reactions. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. Catalysts function by providing an alternate. The two reaction diagrams here represent the same reaction: One without a catalyst and. Catalyst Equilibrium Graph.
From www.youtube.com
Equilibrium Graphs grade 12 Catalyst YouTube Catalyst Equilibrium Graph This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Reaction diagrams for catalyzed reactions. Catalysts function by providing an alternate. This page describes and explains the way that adding a catalyst affects the rate of a reaction. It assumes that you are already familiar with. The effect of catalysts on reaction rates. This graph compares the reaction. Catalyst Equilibrium Graph.
From www.researchgate.net
Effect of catalyst on energy diagram profile. Download Scientific Diagram Catalyst Equilibrium Graph The two reaction diagrams here represent the same reaction: Catalysts function by providing an alternate. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. A catalyst speeds up both the forward and the reverse reactions, so there is no. Catalyst Equilibrium Graph.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Equilibrium Graph The only effect of the. It assumes that you are already familiar with. Catalysts function by providing an alternate. Catalysts can be homogenous (in the same phase as the reactants) or. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. A catalyst speeds up both. Catalyst Equilibrium Graph.
From www.i-ciencias.com
físicoquímica ¿Que diagrama muestra el efecto de la Catalyst Equilibrium Graph Catalysts can be homogenous (in the same phase as the reactants) or. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Catalysts function by providing an alternate. The only effect of the. Reaction diagrams for catalyzed reactions. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates.. Catalyst Equilibrium Graph.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID2633301 Catalyst Equilibrium Graph The two reaction diagrams here represent the same reaction: Catalysts function by providing an alternate. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. The effect of catalysts on reaction rates. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Reaction diagrams for catalyzed reactions. One without a catalyst and one with a. Catalysts. Catalyst Equilibrium Graph.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID6050390 Catalyst Equilibrium Graph The two reaction diagrams here represent the same reaction: Catalysts function by providing an alternate. Catalysts function by providing an alternate. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. It assumes that you are already familiar. Catalyst Equilibrium Graph.
From 2012books.lardbucket.org
Chemical Equilibrium Catalyst Equilibrium Graph Catalysts function by providing an alternate. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. One without a catalyst and one with a. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. The effect of catalysts on reaction rates. The two reaction diagrams here represent the same reaction: Catalysts can be homogenous (in the. Catalyst Equilibrium Graph.
From www.slideserve.com
PPT Chapter 12 Chemical Equilibrium PowerPoint Presentation, free download ID6308959 Catalyst Equilibrium Graph Catalysts function by providing an alternate. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. This page describes and explains the way that adding a catalyst affects the rate of a reaction. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Catalysts function by providing an. Catalyst Equilibrium Graph.
From circuitdbplastered.z13.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Equilibrium Graph The effect of catalysts on reaction rates. The two reaction diagrams here represent the same reaction: Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. The only effect of the. Catalysts can be homogenous (in the same phase as the reactants) or. Catalysts function by providing an alternate. One without a. Catalyst Equilibrium Graph.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst. Download Scientific Catalyst Equilibrium Graph It assumes that you are already familiar with. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. One without a catalyst and one with a. Reaction diagrams for catalyzed reactions. Catalysts function by providing an alternate. Catalysts can be homogenous (in the same phase as the reactants) or. This graph compares. Catalyst Equilibrium Graph.
From sheetalschemblog.blogspot.com
Sheetal's Chemistry Blog 6.2.5,6.2.6 and 6.2.7 Catalyst Equilibrium Graph It assumes that you are already familiar with. This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Catalysts function by providing an alternate. One without a catalyst and one with a. A catalyst speeds up both the forward and the reverse reactions, so there is no uneven change in reaction rates. The only effect of the. Reaction. Catalyst Equilibrium Graph.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID2601311 Catalyst Equilibrium Graph Catalysts function by providing an alternate. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. The two reaction diagrams here represent the same reaction: This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. One without a catalyst and one with a. The effect of catalysts on reaction rates.. Catalyst Equilibrium Graph.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID5967535 Catalyst Equilibrium Graph This graph compares the reaction coordinates for catalyzed and uncatalyzed alkene hydrogenation. Catalysts affect the rate of a chemical reaction by altering its mechanism to provide a lower activation energy. The only effect of the. Reaction diagrams for catalyzed reactions. The two reaction diagrams here represent the same reaction: This page describes and explains the way that adding a catalyst. Catalyst Equilibrium Graph.