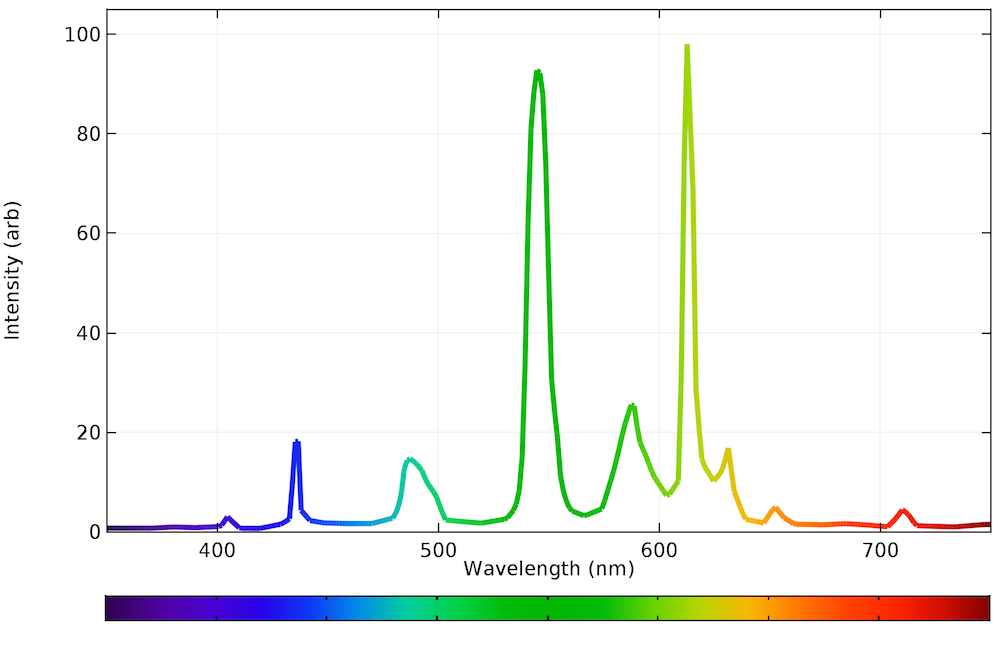
Emission Line Spectrometry . The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. The number of photons emitted is proportional to the number of atoms. The atomic emission spectrum is composed of discrete spectral lines. Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. In classical emission spectrometry, there are numerous listings of spectral lines. For instance, massachusetts institute of technology (mit). The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations.
from www.comsol.com
The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. The number of photons emitted is proportional to the number of atoms. Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. The atomic emission spectrum is composed of discrete spectral lines. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. In classical emission spectrometry, there are numerous listings of spectral lines. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. For instance, massachusetts institute of technology (mit).
Calculating the Emission Spectra from Common Light Sources COMSOL Blog
Emission Line Spectrometry The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. In classical emission spectrometry, there are numerous listings of spectral lines. For instance, massachusetts institute of technology (mit). The number of photons emitted is proportional to the number of atoms. Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. The atomic emission spectrum is composed of discrete spectral lines. The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an.
From griffithobservatory.org
Spectroscope Griffith Observatory Southern California’s gateway to Emission Line Spectrometry The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. In classical emission spectrometry, there are numerous listings of spectral lines. The number of. Emission Line Spectrometry.
From www.youtube.com
Atomic Emission Spectroscopy YouTube Emission Line Spectrometry The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. The number of photons emitted is proportional to the number of atoms. The atomic emission spectrum is composed of discrete. Emission Line Spectrometry.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Line Spectrometry In classical emission spectrometry, there are numerous listings of spectral lines. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. For instance, massachusetts institute of technology (mit). The number of photons emitted is proportional to the number of atoms. The atomic emission spectrum is composed of. Emission Line Spectrometry.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Line Spectrometry The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. In classical emission spectrometry, there are numerous listings of spectral lines. The number of photons emitted is proportional to the number of atoms. For instance, massachusetts institute of technology (mit). The atomic emission spectrum is composed of. Emission Line Spectrometry.
From www.youtube.com
Inductively coupled plasma optical emission spectroscopy (ICPOES Emission Line Spectrometry Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. For instance, massachusetts institute of technology. Emission Line Spectrometry.
From mavink.com
Visible Line Spectrum Of Hydrogen Emission Line Spectrometry In classical emission spectrometry, there are numerous listings of spectral lines. The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. For instance, massachusetts institute of technology (mit). Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. The atomic emission spectrum is composed of discrete spectral lines.. Emission Line Spectrometry.
From koreasci.com
자료실 수소원자의 스펙트럼 Emission Line Spectrometry The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. The number of photons emitted is proportional to the number of atoms. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. The atomic. Emission Line Spectrometry.
From scienceatyourdoorstep.com
The Atomic Spectrum Science at Your Doorstep Emission Line Spectrometry Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. For instance, massachusetts institute of technology (mit). The atomic emission spectrum is composed of discrete spectral lines. The easiest approach to selecting a wavelength is to record. Emission Line Spectrometry.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Emission Line Spectrometry The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. In classical emission spectrometry, there are numerous listings of spectral lines. The atomic emission spectrum is composed of discrete spectral lines.. Emission Line Spectrometry.
From studymind.co.uk
Flame Emission Spectroscopy (GCSE Chemistry) Study Mind Emission Line Spectrometry The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. For instance, massachusetts institute of technology (mit). Finally, ba has a single strong emission. Emission Line Spectrometry.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Line Spectrometry The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. The atomic emission spectrum is composed of discrete spectral lines. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. Mg exhibits three closely. Emission Line Spectrometry.
From medium.com
Light and Dark Understanding Spectrometry by Amelia Settembre Medium Emission Line Spectrometry In classical emission spectrometry, there are numerous listings of spectral lines. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. For instance, massachusetts institute of technology (mit). The number of photons. Emission Line Spectrometry.
From chem.libretexts.org
4.2 Understanding Atomic Spectra Chemistry LibreTexts Emission Line Spectrometry In classical emission spectrometry, there are numerous listings of spectral lines. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. The number of photons emitted is proportional to the number of atoms. Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604. Emission Line Spectrometry.
From chem.libretexts.org
14.1 Vocabulary Chemistry LibreTexts Emission Line Spectrometry For instance, massachusetts institute of technology (mit). Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. The number of photons emitted is proportional to the number of atoms. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. The emission. Emission Line Spectrometry.
From www.youtube.com
2.2 Hydrogen emission spectrum (SL) YouTube Emission Line Spectrometry Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. The atomic emission spectrum is composed of discrete spectral lines. Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. In classical. Emission Line Spectrometry.
From www.slideserve.com
PPT Principle of Emission Spectroscopy I PowerPoint Presentation Emission Line Spectrometry The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. In classical emission spectrometry, there are numerous listings of spectral lines. The number of photons emitted is proportional to the number of atoms. For instance, massachusetts institute of technology (mit). The easiest approach to selecting a wavelength is to record the sample’s emission. Emission Line Spectrometry.
From chem.libretexts.org
11.4 Bohr's Theory of the Hydrogen Emission Spectrum Chemistry Emission Line Spectrometry The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. For instance, massachusetts institute of technology (mit). The atomic emission spectrum is composed of discrete spectral lines. Finally, ba has a single. Emission Line Spectrometry.
From www.pinterest.ca
Pin de Sandra Pedro em Physics is awesome Astrofísica, Quimica geral Emission Line Spectrometry The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. The number of photons emitted is proportional to the number of atoms. For instance, massachusetts institute of technology (mit). Mg exhibits three closely spaced emission lines at. Emission Line Spectrometry.
From joihsprvv.blob.core.windows.net
Emission Spectra Bbc Bitesize at Jose Doty blog Emission Line Spectrometry For instance, massachusetts institute of technology (mit). Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. The number of photons emitted is proportional to the number of atoms. Mg exhibits three closely spaced emission lines at. Emission Line Spectrometry.
From www.optecks.com
Spectroscopy Introduction and Traditional Methods Emission Line Spectrometry Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. In classical emission spectrometry, there are numerous listings of spectral lines. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an. Emission Line Spectrometry.
From www.pinterest.com
Absorption spectrum of the elements spectroscopy Physics, Earth and Emission Line Spectrometry The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. In classical emission spectrometry, there are numerous listings of spectral lines. The emission lines. Emission Line Spectrometry.
From www.slideshare.net
Atomic Spectroscopy Basic Principles and Instruments Emission Line Spectrometry Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. For instance, massachusetts institute of technology (mit). In classical emission spectrometry, there are numerous listings of spectral lines. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. Finally, ba has a. Emission Line Spectrometry.
From cecyvdro.blob.core.windows.net
How Is Spectroscopy Used at William Dixon blog Emission Line Spectrometry Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. The atomic emission spectrum is composed of discrete spectral lines. For instance, massachusetts institute of technology (mit). The number of photons emitted is proportional to the number of atoms. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look. Emission Line Spectrometry.
From www.researchgate.net
Optical emission spectroscopy of hydrogen plasma during reduction of Emission Line Spectrometry The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. The number of photons emitted is proportional to the number of atoms. In classical emission spectrometry, there are numerous listings of. Emission Line Spectrometry.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Emission Line Spectrometry The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. For instance, massachusetts institute. Emission Line Spectrometry.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum Emission Line Spectrometry Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. For instance, massachusetts institute of technology (mit). The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and. Emission Line Spectrometry.
From hubblesite.org
Spectroscopy Reading the Rainbow Emission Line Spectrometry In classical emission spectrometry, there are numerous listings of spectral lines. Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. For instance, massachusetts institute of technology (mit). Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. The atomic emission spectrum is composed of discrete spectral lines. The. Emission Line Spectrometry.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Line Spectrometry For instance, massachusetts institute of technology (mit). Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. In classical emission spectrometry, there are numerous listings of spectral lines. The number of photons emitted is proportional to the number of. Emission Line Spectrometry.
From klafajwah.blob.core.windows.net
Spectroscopy Absorbance Wavelength at Gertrude Peters blog Emission Line Spectrometry Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. The easiest approach to selecting a wavelength is to record the sample’s. Emission Line Spectrometry.
From www.reddit.com
Lightfall CE Mini Lithograph Mystery r/raidsecrets Emission Line Spectrometry The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. The atomic emission spectrum is composed of discrete spectral lines. The number of photons emitted is proportional to the number of atoms. In classical emission spectrometry, there are numerous listings of spectral lines. For instance, massachusetts institute. Emission Line Spectrometry.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Emission Line Spectrometry The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. The number of photons emitted is proportional to the number of atoms. Mg exhibits three closely spaced emission lines at. Emission Line Spectrometry.
From es.slideshare.net
Emission spectroscopy Emission Line Spectrometry Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. The easiest approach to selecting a. Emission Line Spectrometry.
From klazdmetw.blob.core.windows.net
Line Spectra How Does It Work at Lindsey Robinson blog Emission Line Spectrometry For instance, massachusetts institute of technology (mit). Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. Finally, ba has a single strong emission line at 553.5481 nm, but also many less intense. The number of photons emitted. Emission Line Spectrometry.
From www.pinterest.co.uk
Image of absorption, emission, and continuous spectra. Absorption Emission Line Spectrometry Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. In classical emission spectrometry, there are numerous listings of spectral lines. For instance, massachusetts institute of technology (mit). The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look for an emission line that provides an. The number of photons. Emission Line Spectrometry.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Emission Line Spectrometry The emission lines are usually determined photographically specially in case of electric spark or electric arc excitations. Mg exhibits three closely spaced emission lines at 516.7322 nm, 517.2684 nm, and 518.3604 nm. The number of photons emitted is proportional to the number of atoms. The easiest approach to selecting a wavelength is to record the sample’s emission spectrum and look. Emission Line Spectrometry.