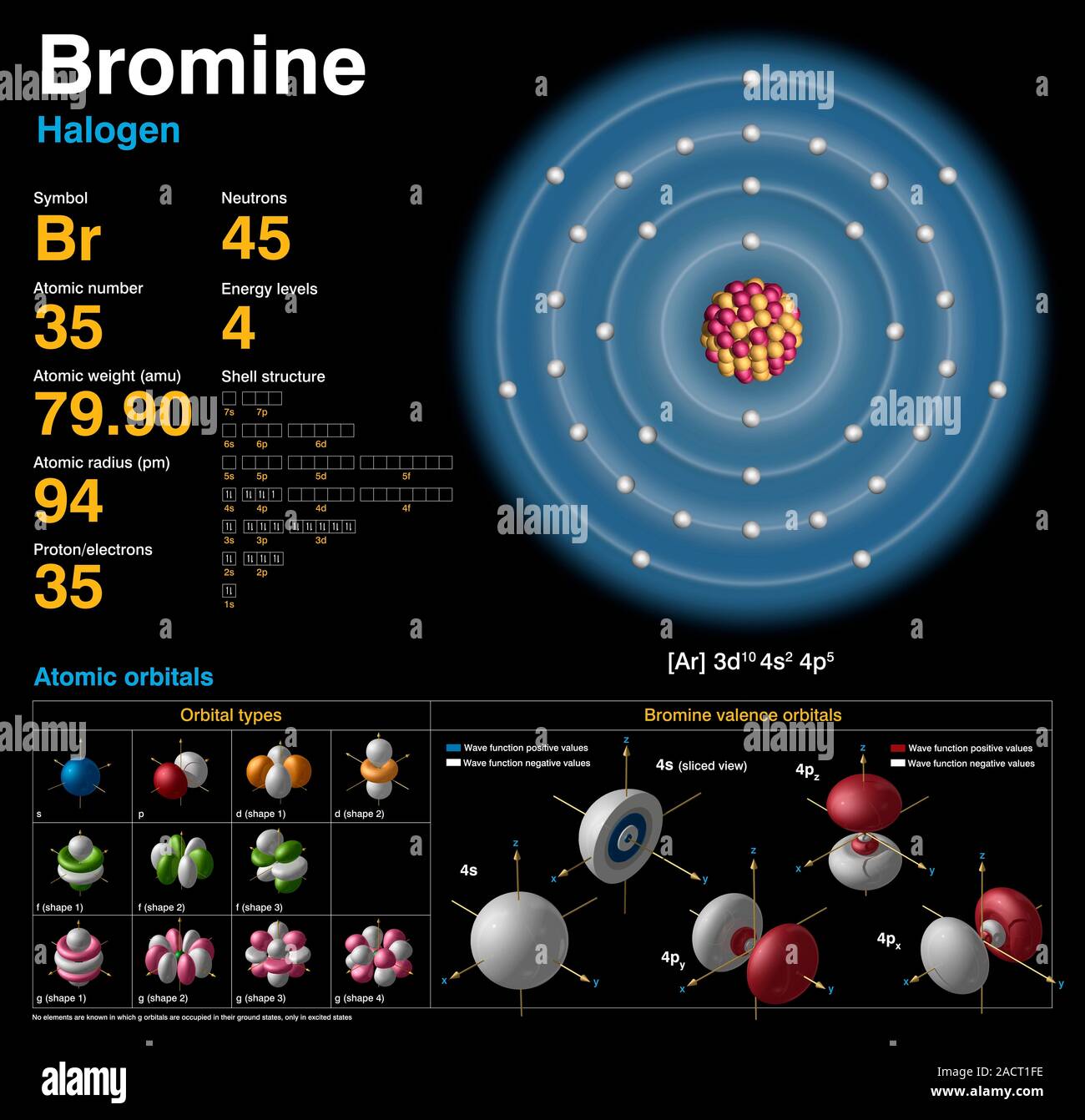
Bromine Dioxide Valence Electrons . ⇒ total number of the valence electrons in oxygen = 6. By looking at the periodic table, we get to know, that bromine belongs to the 17th periodic group and oxygen to the 16th. Bromine is a group viia element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are. Iron is a transition metal, so the number. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Hence, the valence electron for bromine is 7 and for oxygen, it is 6. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Bromine has seven valence electrons. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen.
from ar.inspiredpencil.com
Iron is a transition metal, so the number. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Bromine is a group viia element in the periodic table and contains seven electrons in its last shell. Hence, the valence electron for bromine is 7 and for oxygen, it is 6. Bromine has seven valence electrons. ⇒ total number of the valence electrons in oxygen = 6. Now, we know how many electrons are. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. By looking at the periodic table, we get to know, that bromine belongs to the 17th periodic group and oxygen to the 16th.
Orbital Diagram Of Bromine
Bromine Dioxide Valence Electrons Iron is a transition metal, so the number. Bromine has seven valence electrons. Hence, the valence electron for bromine is 7 and for oxygen, it is 6. Bromine is a group viia element in the periodic table and contains seven electrons in its last shell. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. Iron is a transition metal, so the number. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Now, we know how many electrons are. By looking at the periodic table, we get to know, that bromine belongs to the 17th periodic group and oxygen to the 16th. ⇒ total number of the valence electrons in oxygen = 6.
From www.numerade.com
The compound below is treated with Nbromosuccinimide (NBS) in the Bromine Dioxide Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Hence, the valence electron for bromine is 7 and for oxygen, it is 6. Bromine has seven valence electrons. Now, we know how many electrons are. ⇒ total number of the valence electrons in oxygen = 6. Iron is. Bromine Dioxide Valence Electrons.
From exobbalij.blob.core.windows.net
Bromine Atom Electrons at Paula McCullough blog Bromine Dioxide Valence Electrons Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. Iron is a transition metal, so the number. Now, we know how many electrons are. Bromine has seven valence electrons. By looking at the periodic table, we get to know, that bromine belongs to the 17th periodic group and oxygen to the 16th. Hence, the. Bromine Dioxide Valence Electrons.
From www.youtube.com
How many valence electrons does bromine have? YouTube Bromine Dioxide Valence Electrons By looking at the periodic table, we get to know, that bromine belongs to the 17th periodic group and oxygen to the 16th. ⇒ total number of the valence electrons in oxygen = 6. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. 93 rows you may assume the valences of the chemical elements—the. Bromine Dioxide Valence Electrons.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Bromine Dioxide Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Now, we know how many electrons are. By looking at the periodic. Bromine Dioxide Valence Electrons.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Dioxide Valence Electrons ⇒ total number of the valence electrons in oxygen = 6. Bromine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Now, we know how many electrons are. Hence, the valence electron for bromine is 7 and for oxygen, it is 6. The electron. Bromine Dioxide Valence Electrons.
From exooeohnk.blob.core.windows.net
Bromine Total Number Of Valence Electrons at Rebeca Henshaw blog Bromine Dioxide Valence Electrons Bromine has seven valence electrons. ⇒ total number of the valence electrons in oxygen = 6. By looking at the periodic table, we get to know, that bromine belongs to the 17th periodic group and oxygen to the 16th. Bromine is a group viia element in the periodic table and contains seven electrons in its last shell. Iron is a. Bromine Dioxide Valence Electrons.
From www.numerade.com
SOLVED The compound below is treated with Nbromosuccinimide (NBS) in Bromine Dioxide Valence Electrons Iron is a transition metal, so the number. By looking at the periodic table, we get to know, that bromine belongs to the 17th periodic group and oxygen to the 16th. Bromine has seven valence electrons. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. 93 rows you may assume the valences of the. Bromine Dioxide Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does CO2 (Carbon Dioxide) Have? Bromine Dioxide Valence Electrons Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. Bromine is a group viia element in the periodic table and contains seven electrons in its last shell. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6.. Bromine Dioxide Valence Electrons.
From www.chegg.com
Solved 1 View the first formula in Data Table 1. a Determine Bromine Dioxide Valence Electrons ⇒ total number of the valence electrons in oxygen = 6. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. By looking at the periodic table, we get to know, that bromine belongs to the 17th periodic group and oxygen to the. Bromine Dioxide Valence Electrons.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Bromine Dioxide Valence Electrons Iron is a transition metal, so the number. By looking at the periodic table, we get to know, that bromine belongs to the 17th periodic group and oxygen to the 16th. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Now, we. Bromine Dioxide Valence Electrons.
From iperiodictable.com
How Can We Find A Electron Configuration For Bromine (Br) Bromine Dioxide Valence Electrons Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s. Bromine Dioxide Valence Electrons.
From www.numerade.com
SOLVED What is the valence electron configuration for the bromine atom? Bromine Dioxide Valence Electrons Bromine has seven valence electrons. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Now, we know how many electrons are. 93 rows you may assume the. Bromine Dioxide Valence Electrons.
From periodictable.me
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram Bromine Dioxide Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Iron is a transition metal, so the number. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. By looking at the periodic table, we get to know, that bromine belongs to the 17th. Bromine Dioxide Valence Electrons.
From proper-cooking.info
Bromine Valence Electrons Bromine Dioxide Valence Electrons Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. ⇒ total number of the valence electrons in oxygen = 6. Now, we know how many electrons are. Hence, the valence electron for bromine is 7 and for oxygen, it is 6. By looking at the periodic table, we get to know, that bromine belongs. Bromine Dioxide Valence Electrons.
From www.youtube.com
How many valence electrons does bromine have? YouTube Bromine Dioxide Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. ⇒ total number of the valence electrons in oxygen = 6. Hence,. Bromine Dioxide Valence Electrons.
From www.anyrgb.com
Pblock, homonuclear Molecule, bromine Dioxide, iodine127, diatomic Bromine Dioxide Valence Electrons Bromine has seven valence electrons. Bromine is a group viia element in the periodic table and contains seven electrons in its last shell. Hence, the valence electron for bromine is 7 and for oxygen, it is 6. Now, we know how many electrons are. ⇒ total number of the valence electrons in oxygen = 6. Iron is a transition metal,. Bromine Dioxide Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Oxygen Difluoride Have? Bromine Dioxide Valence Electrons Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. ⇒ total number of the valence electrons in oxygen = 6. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Bromine has seven valence electrons. Bromine is. Bromine Dioxide Valence Electrons.
From www.numerade.com
SOLVED For the Bromine atom a) Determine the total number of unpaired Bromine Dioxide Valence Electrons Bromine has seven valence electrons. Hence, the valence electron for bromine is 7 and for oxygen, it is 6. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. Now, we know how many. Bromine Dioxide Valence Electrons.
From nikitakrystyna.blogspot.com
21+ bromine bohr diagram NikitaKrystyna Bromine Dioxide Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Hence, the valence electron for bromine is 7 and for oxygen, it. Bromine Dioxide Valence Electrons.
From ar.inspiredpencil.com
Electron Configuration Of Bromine Bromine Dioxide Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. By looking at the periodic table, we get to know, that bromine. Bromine Dioxide Valence Electrons.
From phootoscelebrities.com
How many valence electrons does bromine have? Bromine Dioxide Valence Electrons ⇒ total number of the valence electrons in oxygen = 6. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Bromine has seven valence electrons. Iron is a transition metal, so the number. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen.. Bromine Dioxide Valence Electrons.
From www.chegg.com
Solved How many pairs of valence electrons do the bromine Bromine Dioxide Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Now, we know how many electrons are. Bromine has seven valence electrons. Hence, the valence electron for bromine is 7 and for oxygen, it is 6. By looking at the periodic table, we. Bromine Dioxide Valence Electrons.
From iperiodictable.com
How Can We Find A Electron Configuration For Bromine (Br) Bromine Dioxide Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. Now, we know how many electrons are. By looking at the periodic table, we get to know, that bromine belongs to the 17th periodic. Bromine Dioxide Valence Electrons.
From www.chegg.com
Solved How many valence electrons are there in the bromine Bromine Dioxide Valence Electrons Iron is a transition metal, so the number. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Hence, the valence electron for bromine is 7 and for oxygen, it is 6. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. Bromine is. Bromine Dioxide Valence Electrons.
From www.alamy.com
Bromine, Br, periodic table element with name, symbol, atomic number Bromine Dioxide Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Bromine is a group viia element in the periodic table and contains seven electrons in its last shell. Iron is a transition metal, so the number. By looking at the periodic table, we get to know, that bromine belongs. Bromine Dioxide Valence Electrons.
From chem.libretexts.org
11.6 Delocalized Electrons Bonding in the Benzene Molecule Bromine Dioxide Valence Electrons Bromine has seven valence electrons. Iron is a transition metal, so the number. Now, we know how many electrons are. Hence, the valence electron for bromine is 7 and for oxygen, it is 6. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. By looking at the periodic table, we get to know, that. Bromine Dioxide Valence Electrons.
From phootoscelebrities.com
How Many Valence Electrons Does Bromine Have? Bromine Dioxide Valence Electrons Bromine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Bromine is a group viia element in. Bromine Dioxide Valence Electrons.
From exycbirkc.blob.core.windows.net
Is Bromine An Atom Or Ion at Nancy Dunmire blog Bromine Dioxide Valence Electrons Bromine has seven valence electrons. Iron is a transition metal, so the number. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. ⇒ total number of the valence electrons in oxygen = 6. By looking at the periodic table, we get to know, that bromine belongs to the 17th periodic group and oxygen to. Bromine Dioxide Valence Electrons.
From angelokruwpetty.blogspot.com
How Many Valence Electrons in Bromine AngelokruwPetty Bromine Dioxide Valence Electrons Hence, the valence electron for bromine is 7 and for oxygen, it is 6. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. Bromine has seven valence electrons. ⇒ total number of the valence electrons in oxygen = 6. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s. Bromine Dioxide Valence Electrons.
From www.alamyimages.fr
Schéma d'électrons et de symbole pour le brome illustration Image Bromine Dioxide Valence Electrons The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. Bromine has seven valence electrons. By looking at the periodic table, we get to know, that bromine belongs to the 17th periodic group and oxygen to the 16th. Iron is a transition metal,. Bromine Dioxide Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for SO2 (Sulfur Dioxide)? Bromine Dioxide Valence Electrons Hence, the valence electron for bromine is 7 and for oxygen, it is 6. Iron is a transition metal, so the number. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or. Bromine Dioxide Valence Electrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Dioxide Valence Electrons ⇒ total number of the valence electrons in oxygen = 6. Iron is a transition metal, so the number. Bromine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. By looking at the periodic table, we get to know, that bromine belongs to the. Bromine Dioxide Valence Electrons.
From ar.inspiredpencil.com
Orbital Diagram Of Bromine Bromine Dioxide Valence Electrons Iron is a transition metal, so the number. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. ⇒ total number of the valence electrons in oxygen = 6. Bromine has seven valence electrons. Now, we know how many electrons are. By looking at the periodic table, we get. Bromine Dioxide Valence Electrons.
From exobbalij.blob.core.windows.net
Bromine Atom Electrons at Paula McCullough blog Bromine Dioxide Valence Electrons Bromine has seven valence electrons. Bromine (br) is the central atom in bro2 because it is less electronegative than oxygen. ⇒ total number of the valence electrons in oxygen = 6. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Iron is a transition metal, so the number.. Bromine Dioxide Valence Electrons.
From diagramlilythebunny76.z22.web.core.windows.net
Dot And Cross Diagram For Carbon Dioxide Bromine Dioxide Valence Electrons Now, we know how many electrons are. The electron configuration of an iron atom is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6 or [ar]4s 2 3d 6. By looking at the periodic table, we get to know, that bromine belongs to the 17th periodic group and oxygen to the 16th. Hence, the valence. Bromine Dioxide Valence Electrons.