Zinc Colour Reaction . \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. The reactivity series of metals is a chart listing metals in order of decreasing reactivity. the colors of a transition metal ion depend on its conditions in a chemical solution, but some colors are good to know (especially if you're taking ap chemistry): Zn + i 2 → zni 2 Sulfur has a strong affinity for zinc. These types of reactions are called direct redox. metallic zinc reacts with weak acids very slowly. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. When heated, the two powders react. the colours of complex metal ions. Zinc powder is added to a solution of iodine in ethanol. A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2:
from blog.thepipingmart.com
zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: metallic zinc reacts with weak acids very slowly. When heated, the two powders react. Zinc powder is added to a solution of iodine in ethanol. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). Zn + i 2 → zni 2 These types of reactions are called direct redox. ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. Sulfur has a strong affinity for zinc. A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test.
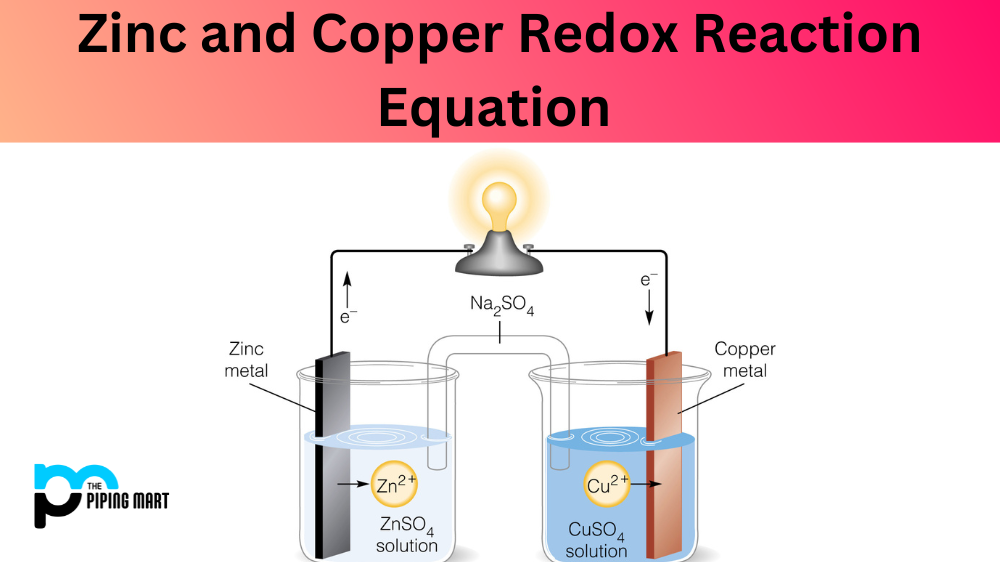
Zinc and Copper Redox Reaction Equation
Zinc Colour Reaction Zn + i 2 → zni 2 ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. Sulfur has a strong affinity for zinc. When heated, the two powders react. A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test. metallic zinc reacts with weak acids very slowly. Zinc powder is added to a solution of iodine in ethanol. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zn + i 2 → zni 2 An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. the colors of a transition metal ion depend on its conditions in a chemical solution, but some colors are good to know (especially if you're taking ap chemistry): \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). the colours of complex metal ions. These types of reactions are called direct redox. The reactivity series of metals is a chart listing metals in order of decreasing reactivity.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc Colour Reaction the colours of complex metal ions. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Sulfur has a strong affinity for zinc. Zinc powder is added to a solution of iodine in ethanol. ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. Zn. Zinc Colour Reaction.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Zinc Colour Reaction ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. Zinc powder is added to a solution of iodine in ethanol. A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test. metallic zinc reacts with weak acids very. Zinc Colour Reaction.
From www.researchgate.net
Simplified configuration of zinc reactions with water Download Zinc Colour Reaction zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test. Zn + i 2 → zni 2 ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction.. Zinc Colour Reaction.
From www.youtube.com
Copper (II) sulfate and zinc reactions YouTube Zinc Colour Reaction A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test. These types of reactions are called direct redox. Sulfur has a strong affinity for zinc. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). the colors of a transition metal ion depend on its conditions in a chemical solution, but some colors are good. Zinc Colour Reaction.
From www.youtube.com
Zinc metal reaction Nitric acid reaction Zinc in nitric acid Zinc Colour Reaction An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zn + i 2 → zni 2 Sulfur has a strong affinity for zinc. the colours of complex metal ions. metallic zinc reacts with weak acids very slowly. A related phenomenon is the emission spectra of transition metal salts, used to identify. Zinc Colour Reaction.
From www.youtube.com
Zinc and Copper II Chloride YouTube Zinc Colour Reaction An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). the colors of a transition metal ion depend on its conditions in a chemical solution, but some colors are good to know (especially if you're taking ap chemistry): Zn + i 2 → zni 2 A related phenomenon. Zinc Colour Reaction.
From colorscombo.com
What Is The Color Of Zinc Zinc Colour Reaction A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zn + i 2 → zni 2 Zinc powder is added to a solution of iodine in ethanol. Sulfur has a strong affinity for zinc.. Zinc Colour Reaction.
From www.youtube.com
Colorchanging Zinc Oxide and submit your questions for Ben YouTube Zinc Colour Reaction Zinc powder is added to a solution of iodine in ethanol. Sulfur has a strong affinity for zinc. metallic zinc reacts with weak acids very slowly. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zn + i 2 → zni 2 An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating. Zinc Colour Reaction.
From www.alamy.com
Reaction of zinc with hydrochloric acid Stock Photo Alamy Zinc Colour Reaction A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test. When heated, the two powders react. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. The reactivity series of metals is a chart listing metals in order of decreasing reactivity. the colors of. Zinc Colour Reaction.
From phys.org
Bringing out the color in zinc to expand its potential properties Zinc Colour Reaction ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. These types of reactions are called direct redox. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: The reactivity series of metals is a chart listing metals in order of decreasing reactivity. A related phenomenon. Zinc Colour Reaction.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Zinc Colour Reaction \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). Zn + i 2 → zni 2 An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. These types of reactions are called direct redox. When heated, the two powders react. the colours of complex metal ions. the colors of a transition metal ion depend on its. Zinc Colour Reaction.
From www.sciencephoto.com
Copper oxide and zinc reaction Stock Image A500/0682 Science Zinc Colour Reaction When heated, the two powders react. metallic zinc reacts with weak acids very slowly. Sulfur has a strong affinity for zinc. A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test. Zinc powder is added to a solution of iodine in ethanol. Zn + i 2 → zni 2 . Zinc Colour Reaction.
From www.youtube.com
Réaction entre le zinc et l'acide sulfurique, catalysée par le cuivre Zinc Colour Reaction An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. Zn + i 2 → zni 2 The reactivity series of metals is a chart listing metals in order of decreasing reactivity. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). ions of any metal that is below zinc, such as lead or silver, would oxidize the. Zinc Colour Reaction.
From blog.thepipingmart.com
Exploring the Different Reactions of Zinc to Acetic Acid and Copper Zinc Colour Reaction metallic zinc reacts with weak acids very slowly. The reactivity series of metals is a chart listing metals in order of decreasing reactivity. Zn + i 2 → zni 2 A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test. Sulfur has a strong affinity for zinc. the colours. Zinc Colour Reaction.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Zinc Colour Reaction When heated, the two powders react. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc powder is added to a solution of iodine in ethanol. Zn + i 2 → zni 2 These types of reactions are called direct redox. Sulfur has a strong affinity for zinc. ions of any. Zinc Colour Reaction.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc Colour Reaction Zinc powder is added to a solution of iodine in ethanol. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: metallic zinc reacts with weak acids very slowly. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. the colors of a transition metal ion depend on its conditions. Zinc Colour Reaction.
From www.youtube.com
Reaction of Zinc Sulphate (ZnSO4) with Calcium hydroxide (Ca(OH)2 Zinc Colour Reaction A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test. Sulfur has a strong affinity for zinc. Zn + i 2 → zni 2 zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: ions of any metal that is below zinc, such as lead or silver, would. Zinc Colour Reaction.
From colorscombo.com
What Color Is Zinc Zinc Colour Reaction zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: the colours of complex metal ions. When heated, the two powders react. The reactivity series of metals is a chart listing metals in order of decreasing reactivity. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. These types of reactions. Zinc Colour Reaction.
From www.youtube.com
Reaction Of Zinc with Hydrochloric acid Chemistry demonstration YouTube Zinc Colour Reaction ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. Sulfur has a strong affinity for zinc. the colors of a transition metal ion depend on its conditions in a chemical solution, but some colors are good to know (especially if you're taking ap chemistry): When heated,. Zinc Colour Reaction.
From dxovatzox.blob.core.windows.net
Zinc Hydroxide Color at Thelma Collins blog Zinc Colour Reaction When heated, the two powders react. These types of reactions are called direct redox. the colours of complex metal ions. metallic zinc reacts with weak acids very slowly. The reactivity series of metals is a chart listing metals in order of decreasing reactivity. ions of any metal that is below zinc, such as lead or silver, would. Zinc Colour Reaction.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Zinc Colour Reaction metallic zinc reacts with weak acids very slowly. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). Sulfur has a strong affinity for zinc. Zinc powder is added to a solution of iodine in ethanol. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. the colors of a transition metal ion depend on its conditions. Zinc Colour Reaction.
From www.slideshare.net
Replacement reactions Zinc Colour Reaction The reactivity series of metals is a chart listing metals in order of decreasing reactivity. A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test. metallic zinc reacts with weak acids very slowly. the colours of complex metal ions. Sulfur has a strong affinity for zinc. \[\ce{zn^{2+}(aq) + 2nh3(aq). Zinc Colour Reaction.
From www.youtube.com
A ZincSulfuric Acid Reaction, Dramatized YouTube Zinc Colour Reaction The reactivity series of metals is a chart listing metals in order of decreasing reactivity. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). the colours of complex metal ions. Sulfur has a strong affinity for zinc. ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. An exothermic redox reaction. Zinc Colour Reaction.
From en.ppt-online.org
Metals online presentation Zinc Colour Reaction A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test. ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. the colors of a transition metal ion depend on its conditions in a chemical solution, but some colors. Zinc Colour Reaction.
From thewonderofscience.com
Reaction of zinc and hydrochloric acid (NY) — The Wonder of Science Zinc Colour Reaction the colors of a transition metal ion depend on its conditions in a chemical solution, but some colors are good to know (especially if you're taking ap chemistry): These types of reactions are called direct redox. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zn + i 2 → zni. Zinc Colour Reaction.
From www.researchgate.net
Reaction scheme for the synthesis of zinc metal complexes 3ac Zinc Colour Reaction Sulfur has a strong affinity for zinc. A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test. Zinc powder is added to a solution of iodine in ethanol. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. When heated, the two powders react. The. Zinc Colour Reaction.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc Colour Reaction A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame test. ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. Sulfur has a strong affinity for zinc. the colors of a transition metal ion depend on its conditions. Zinc Colour Reaction.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Zinc Colour Reaction An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. These types of reactions are called direct redox. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: the. Zinc Colour Reaction.
From askfilo.com
The colour of the solution observed after 30 minutes of placing zinc meta.. Zinc Colour Reaction Zn + i 2 → zni 2 Zinc powder is added to a solution of iodine in ethanol. metallic zinc reacts with weak acids very slowly. The reactivity series of metals is a chart listing metals in order of decreasing reactivity. A related phenomenon is the emission spectra of transition metal salts, used to identify them in the flame. Zinc Colour Reaction.
From colorscombo.com
What Color Is Zinc Zinc Colour Reaction An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). When heated, the two powders react. These types of reactions are called direct redox. Sulfur has a strong affinity for zinc. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc powder is added to a. Zinc Colour Reaction.
From www.researchgate.net
Photograph of prepared copper zinc acetate sample, the colour appear Zinc Colour Reaction Zinc powder is added to a solution of iodine in ethanol. The reactivity series of metals is a chart listing metals in order of decreasing reactivity. Zn + i 2 → zni 2 Sulfur has a strong affinity for zinc. When heated, the two powders react. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: the. Zinc Colour Reaction.
From www.alamyimages.fr
L'oxyde de zinc est une formule chimique moléculaire. Infographies du Zinc Colour Reaction Zinc powder is added to a solution of iodine in ethanol. ions of any metal that is below zinc, such as lead or silver, would oxidize the zinc in a similar reaction. Zn + i 2 → zni 2 zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: metallic zinc reacts with weak acids very. Zinc Colour Reaction.
From fsagung.blogspot.com
WARNA ION LOGAM TRANSISI PENJELASAN, PEMBENTUKAN WARNA Zinc Colour Reaction Zn + i 2 → zni 2 metallic zinc reacts with weak acids very slowly. When heated, the two powders react. An exothermic redox reaction occurs, forming zinc iodide, which can be obtained by evaporating the solvent. the colours of complex metal ions. ions of any metal that is below zinc, such as lead or silver, would. Zinc Colour Reaction.
From fineartamerica.com
Zinc Reacting With Hydrochloric Acid Photograph by Martyn F. Chillmaid Zinc Colour Reaction Sulfur has a strong affinity for zinc. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l). The reactivity series of metals is a chart listing metals in order of decreasing reactivity. These types of reactions are called direct redox. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: metallic zinc reacts with weak acids very slowly. the colors of. Zinc Colour Reaction.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells Zinc Colour Reaction the colors of a transition metal ion depend on its conditions in a chemical solution, but some colors are good to know (especially if you're taking ap chemistry): When heated, the two powders react. zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: ions of any metal that is below zinc, such as lead or. Zinc Colour Reaction.