Scientific Review Board . the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. scientific quality and feasibility are part of ethics review by institutional review boards (irbs). the scientific review board (srb) ensures that the scientific question being asked within a protocol is relevant and that the design of the protocol is appropriate to. The scientific management review board (smrb) advises and make recommendations to the hhs secretary and. the code of practice for scientific advisory committees (copsac) covers all aspects of their governance. astrazeneca today announced the creation of a scientific review board that will act independently to assess requests.
from njacts.rbhs.rutgers.edu
The scientific management review board (smrb) advises and make recommendations to the hhs secretary and. the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. scientific quality and feasibility are part of ethics review by institutional review boards (irbs). in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. astrazeneca today announced the creation of a scientific review board that will act independently to assess requests. the code of practice for scientific advisory committees (copsac) covers all aspects of their governance. the scientific review board (srb) ensures that the scientific question being asked within a protocol is relevant and that the design of the protocol is appropriate to.
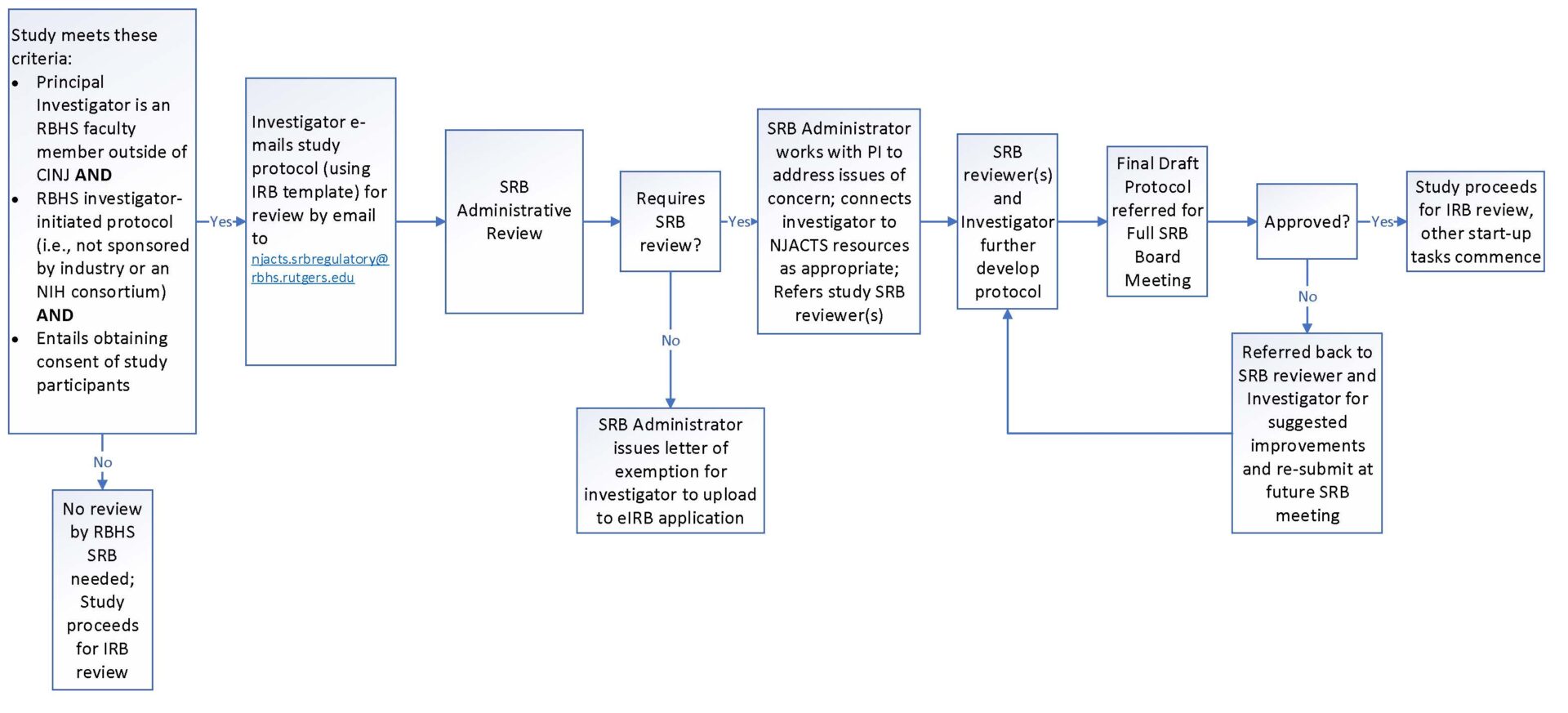
Scientific Review Board New Jersey Alliance for Clinical and
Scientific Review Board The scientific management review board (smrb) advises and make recommendations to the hhs secretary and. in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. the code of practice for scientific advisory committees (copsac) covers all aspects of their governance. The scientific management review board (smrb) advises and make recommendations to the hhs secretary and. scientific quality and feasibility are part of ethics review by institutional review boards (irbs). the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. astrazeneca today announced the creation of a scientific review board that will act independently to assess requests. the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. the scientific review board (srb) ensures that the scientific question being asked within a protocol is relevant and that the design of the protocol is appropriate to.
From inhabitat.com
EPA dismisses 5 members of major scientific review board Scientific Review Board the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. The. Scientific Review Board.
From prabhakarpk.blogspot.com
How to write a good scientific review article Scientific Review Board scientific quality and feasibility are part of ethics review by institutional review boards (irbs). the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. The scientific management review board (smrb) advises and make recommendations to the hhs secretary and. astrazeneca today announced the creation of a. Scientific Review Board.
From ajmp.uwr.edu.pl
Scientific Board Academic Journal of Modern Philology Scientific Review Board The scientific management review board (smrb) advises and make recommendations to the hhs secretary and. in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. the scientific review board (srb) ensures that. Scientific Review Board.
From www.upi.com
EPA dismisses five members of scientific review board Scientific Review Board the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. the scientific review board (srb) ensures that the scientific question being asked within a protocol is relevant and that the design of the protocol is appropriate to. astrazeneca today announced the creation of a scientific review. Scientific Review Board.
From templatelab.com
40 EyeCatching Research Poster Templates (+Scientific Posters) ᐅ Scientific Review Board scientific quality and feasibility are part of ethics review by institutional review boards (irbs). the scientific review board (srb) ensures that the scientific question being asked within a protocol is relevant and that the design of the protocol is appropriate to. the editorial board manages the peer review process and makes final decisions on whether papers should. Scientific Review Board.
From www.pinterest.com
Scientific Measurements Game Print and Digital Science Review Board Scientific Review Board the code of practice for scientific advisory committees (copsac) covers all aspects of their governance. the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. the scientific. Scientific Review Board.
From fbilabqsd.fbi.gov
FRD50204 Disagreements and Scientific Review Boards in Technical Scientific Review Board in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. The scientific management review board (smrb) advises and make recommendations to the hhs secretary and. the scientific review board (srb) ensures that. Scientific Review Board.
From njacts.rbhs.rutgers.edu
Scientific Review Board New Jersey Alliance for Clinical and Scientific Review Board astrazeneca today announced the creation of a scientific review board that will act independently to assess requests. in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. scientific quality and feasibility are part of ethics review by institutional review boards (irbs). the purpose of the scientific management review board. Scientific Review Board.
From www.scribd.com
How to Write a Scientific Review Paper Science Scientific Review Board in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. the code of practice for scientific advisory committees (copsac) covers all aspects of their governance. The scientific management review board (smrb) advises and make recommendations to the hhs secretary and. the editorial board manages the peer review process and makes. Scientific Review Board.
From www.ptglab.com
How to Review a Scientific Paper in 10 Easy Steps Proteintech Group Scientific Review Board scientific quality and feasibility are part of ethics review by institutional review boards (irbs). in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. astrazeneca today announced the creation of a. Scientific Review Board.
From downloaddolitoral.blogspot.com
Scientific Review Summary Examples / Guide on How to Write a Literature Scientific Review Board astrazeneca today announced the creation of a scientific review board that will act independently to assess requests. The scientific management review board (smrb) advises and make recommendations to the hhs secretary and. the code of practice for scientific advisory committees (copsac) covers all aspects of their governance. the editorial board manages the peer review process and makes. Scientific Review Board.
From criticalthinking.cloud
how to do a scientific review of literature Scientific Review Board astrazeneca today announced the creation of a scientific review board that will act independently to assess requests. in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. the. Scientific Review Board.
From downloaddolitoral.blogspot.com
Scientific Review Summary Examples / Guide on How to Write a Literature Scientific Review Board the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. . Scientific Review Board.
From www.slideserve.com
PPT Charter for LBTI Science Review Board PowerPoint Presentation Scientific Review Board the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. . Scientific Review Board.
From studylib.net
Scientific Review Form for Investigator Initiated Studies Scientific Review Board The scientific management review board (smrb) advises and make recommendations to the hhs secretary and. the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. the scientific review board (srb) ensures that the scientific question being asked within a protocol is relevant and that the design of. Scientific Review Board.
From www.postersession.com
Free Powerpoint Scientific Research Poster Templates for Printing Scientific Review Board in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. astrazeneca today announced the creation of a scientific review board that will act independently to assess requests. The scientific management review board (smrb) advises and make recommendations to the hhs secretary and. the code of practice for scientific advisory committees. Scientific Review Board.
From www.cancertodaymag.org
So You Want to Serve on a Scientific Review Panel? Cancer Today Scientific Review Board the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. scientific quality and feasibility are part of ethics review by institutional review boards (irbs). The scientific management review board (smrb) advises and make recommendations to the hhs secretary and. the editorial board manages the peer review. Scientific Review Board.
From sciencenotes.org
Understanding Peer Review in Science Scientific Review Board astrazeneca today announced the creation of a scientific review board that will act independently to assess requests. the scientific review board (srb) ensures that the scientific question being asked within a protocol is relevant and that the design of the protocol is appropriate to. scientific quality and feasibility are part of ethics review by institutional review boards. Scientific Review Board.
From ar.inspiredpencil.com
Scientific Poster Board Scientific Review Board the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. astrazeneca today announced the creation of a scientific review board that will act independently to assess requests. . Scientific Review Board.
From www.pinterest.com
Matter and Chemistry Science Board Game Review Mini Bundle Digital and Scientific Review Board the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. the code of practice for scientific advisory committees (copsac) covers all aspects of their governance. astrazeneca today announced the creation of a scientific review board that will act independently to assess requests. the scientific review board (srb) ensures. Scientific Review Board.
From www.pinterest.com
Science Review Board Game for Grade 5 State Benchmark Exam with 140 Scientific Review Board the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. the code of practice for scientific advisory committees (copsac) covers all aspects of their governance. scientific quality and feasibility are part of ethics review by institutional review boards (irbs). the scientific review board (srb) ensures. Scientific Review Board.
From www.postersession.com
Free Powerpoint Scientific Research Poster Templates for Printing Scientific Review Board astrazeneca today announced the creation of a scientific review board that will act independently to assess requests. scientific quality and feasibility are part of ethics review by institutional review boards (irbs). the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. the code of practice for scientific advisory. Scientific Review Board.
From www.slideserve.com
PPT Charter for LBTI Science Review Board PowerPoint Presentation Scientific Review Board in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. scientific quality and feasibility are part of ethics review by institutional review boards (irbs). the code of practice for scientific advisory committees (copsac) covers all aspects of their governance. astrazeneca today announced the creation of a scientific review board. Scientific Review Board.
From wsbe17hongkong.hk
WSBE17 Hong Kong International Scientific Review Panel Scientific Review Board in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. the code of practice for scientific advisory committees (copsac) covers all aspects of their governance. the scientific review. Scientific Review Board.
From www.thereviewhut.co.uk
Anatomix Science Board Game. Reviews Scientific Review Board the scientific review board (srb) ensures that the scientific question being asked within a protocol is relevant and that the design of the protocol is appropriate to. in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. the purpose of the scientific management review board is to advise hhs and. Scientific Review Board.
From www.getforms.org
Research Poster Template With Abstract Sidebar (48*36) download Scientific Review Board the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. astrazeneca today announced the creation of a scientific review board that will act independently to assess requests. scientific quality and feasibility are part of ethics review by institutional review boards (irbs). the scientific review board. Scientific Review Board.
From studylib.net
Scientific Management Review Board Scientific Review Board scientific quality and feasibility are part of ethics review by institutional review boards (irbs). astrazeneca today announced the creation of a scientific review board that will act independently to assess requests. the scientific review board (srb) ensures that the scientific question being asked within a protocol is relevant and that the design of the protocol is appropriate. Scientific Review Board.
From www.nytimes.com
E.P.A. Dismisses Members of Major Scientific Review Board The New Scientific Review Board the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. the scientific review board (srb) ensures that the scientific question being asked within a protocol is relevant and that the design of the protocol is appropriate to. the code of practice for scientific advisory committees (copsac) covers all aspects. Scientific Review Board.
From phdtalks.org
How to write a scientific review paper PhDTalks Scientific Review Board the code of practice for scientific advisory committees (copsac) covers all aspects of their governance. The scientific management review board (smrb) advises and make recommendations to the hhs secretary and. the scientific review board (srb) ensures that the scientific question being asked within a protocol is relevant and that the design of the protocol is appropriate to. . Scientific Review Board.
From www.pdffiller.com
Fillable Online E.P.A. Dismisses Members of Major Scientific Review Scientific Review Board the scientific review board (srb) ensures that the scientific question being asked within a protocol is relevant and that the design of the protocol is appropriate to. astrazeneca today announced the creation of a scientific review board that will act independently to assess requests. The scientific management review board (smrb) advises and make recommendations to the hhs secretary. Scientific Review Board.
From www.researchgate.net
(PDF) Reviewing is rewarding! Introducing our Scientific Review Board Scientific Review Board scientific quality and feasibility are part of ethics review by institutional review boards (irbs). in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. the purpose of the scientific management review. Scientific Review Board.
From www.enago.com
How to Write a Scientific Review Article Enago Academy Scientific Review Board scientific quality and feasibility are part of ethics review by institutional review boards (irbs). the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. the code of practice for scientific advisory. Scientific Review Board.
From globalresearchjournal.info
Scientific Review Board Global Research Journal Scientific Review Board the editorial board manages the peer review process and makes final decisions on whether papers should be accepted. The scientific management review board (smrb) advises and make recommendations to the hhs secretary and. the scientific review board (srb) ensures that the scientific question being asked within a protocol is relevant and that the design of the protocol is. Scientific Review Board.
From unesdoc.unesco.org
Selection of the formal membership of the IOC Scientific Review Board (SRB) Scientific Review Board the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. the scientific review board (srb) ensures that the scientific question being asked within a protocol is relevant and that the design of the protocol is appropriate to. the editorial board manages the peer review process and. Scientific Review Board.
From studylib.net
Review Board Application Scientific Review Board in the united states (u.s.), research involving human subjects is reviewed by an ethics committee called the. the purpose of the scientific management review board is to advise hhs and nih officials on the use of those organizational. the code of practice for scientific advisory committees (copsac) covers all aspects of their governance. The scientific management review. Scientific Review Board.