Calorimetry Value Definition . calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To do so, the heat is exchanged with a calibrated. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.
from www.tec-science.com
a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do so, the heat is exchanged with a calibrated. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction.
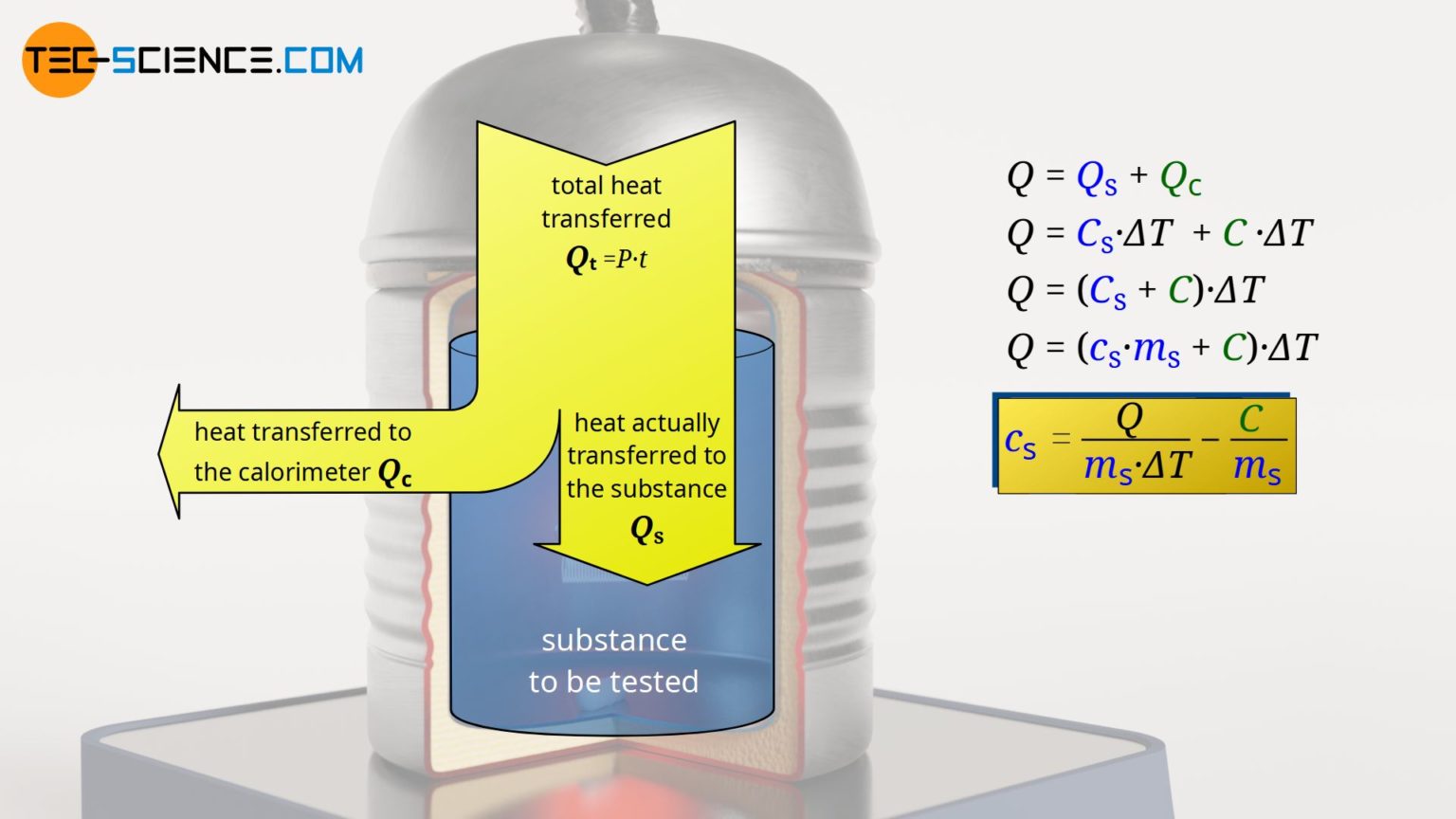
Calorimeter to determine the specific heat capacities of liquids tecscience
Calorimetry Value Definition calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To do so, the heat is exchanged with a calibrated.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Value Definition calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. To do so, the. Calorimetry Value Definition.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimetry Value Definition calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. To do so, the heat is exchanged with a calibrated. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or. Calorimetry Value Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID373573 Calorimetry Value Definition calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the. Calorimetry Value Definition.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimetry Value Definition calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. To do so, the. Calorimetry Value Definition.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Value Definition To do so, the heat is exchanged with a calibrated. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of. Calorimetry Value Definition.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimetry Value Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To do so, the heat is exchanged with a calibrated. calorimetry literally means “heat measurement.” for consistency with other. Calorimetry Value Definition.
From www.youtube.com
Calorimetry calculation YouTube Calorimetry Value Definition calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry. Calorimetry Value Definition.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Calorimetry Value Definition calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is a field of thermochemistry that measures. Calorimetry Value Definition.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimetry Value Definition calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To do so, the heat is exchanged with a calibrated. calorimetry is the process of measuring the amount of heat released. Calorimetry Value Definition.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Value Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. To do so, the heat is exchanged with a calibrated. calorimetry is used to measure amounts of heat transferred to or. Calorimetry Value Definition.
From studylib.net
Calorimetry Calorimetry Value Definition calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. a calorimeter is a device used to measure. Calorimetry Value Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Value Definition calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is a field of. Calorimetry Value Definition.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimetry Value Definition calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. To do so, the. Calorimetry Value Definition.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Value Definition To do so, the heat is exchanged with a calibrated. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. a calorimeter is a device used to measure the amount of heat involved. Calorimetry Value Definition.
From www.youtube.com
Unit 6 Calorimetry YouTube Calorimetry Value Definition calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry literally means “heat measurement.” for. Calorimetry Value Definition.
From www.studypool.com
SOLUTION Chemistry cit u calorimetry definition examples sample problems and etc Studypool Calorimetry Value Definition To do so, the heat is exchanged with a calibrated. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on. Calorimetry Value Definition.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tecscience Calorimetry Value Definition calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry. Calorimetry Value Definition.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Calorimetry Value Definition calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. To do so, the heat is exchanged with a calibrated. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature. Calorimetry Value Definition.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimetry Value Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. To do so, the heat is exchanged with a calibrated. calorimetry is the process of measuring the amount of heat released. Calorimetry Value Definition.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247 Calorimetry Value Definition calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. To do so, the heat is exchanged. Calorimetry Value Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Calorimetry Value Definition calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. calorimetry literally means “heat measurement.” for consistency with. Calorimetry Value Definition.
From quizgrouchiest.z4.web.core.windows.net
Calorimeter Constant Definition Chemistry Calorimetry Value Definition calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. To do so, the heat is exchanged with a calibrated. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is used to measure amounts of heat transferred to or from a. Calorimetry Value Definition.
From www.learner.org
The Energy in Chemical Reactions Thermodynamics and Enthalpy Annenberg Learner Calorimetry Value Definition To do so, the heat is exchanged with a calibrated. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is a field of thermochemistry that measures the amount of heat involved. Calorimetry Value Definition.
From slidetodoc.com
CHEM 1011 Calorimetry The Determination of the Specific Calorimetry Value Definition calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry literally means “heat measurement.” for. Calorimetry Value Definition.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Value Definition calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. calorimetry is used. Calorimetry Value Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID5190363 Calorimetry Value Definition calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. To do so, the heat is exchanged with a calibrated. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry literally means “heat measurement.” for consistency with other forms of. Calorimetry Value Definition.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Value Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. To do so, the heat is exchanged. Calorimetry Value Definition.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle and Structure of Calorimetry Value Definition calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. To do. Calorimetry Value Definition.
From exojtwjwx.blob.core.windows.net
Calorimetry Definition In Your Own Words at Earl Knight blog Calorimetry Value Definition calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. To do. Calorimetry Value Definition.
From cezctooy.blob.core.windows.net
What Is Calorimetry Constant Pressure at Daryl Dirks blog Calorimetry Value Definition calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. calorimetry is used. Calorimetry Value Definition.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types, Examples and Uses CollegeSearch Calorimetry Value Definition calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. To do so, the heat is exchanged with a calibrated. a calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimetry Value Definition.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Value Definition calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. To do so, the heat is exchanged with a calibrated. calorimetry literally means “heat measurement.” for consistency with other forms of. Calorimetry Value Definition.
From exopfwwvc.blob.core.windows.net
Calorimeter In Physics Definition at Laura Addy blog Calorimetry Value Definition calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical. Calorimetry Value Definition.
From www.youtube.com
CALORIMETRY EXAMPLE 3 YouTube Calorimetry Value Definition calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion, the. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device. Calorimetry Value Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Value Definition To do so, the heat is exchanged with a calibrated. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry literally means “heat measurement.” for consistency with other forms of energy and to avoid confusion,. Calorimetry Value Definition.