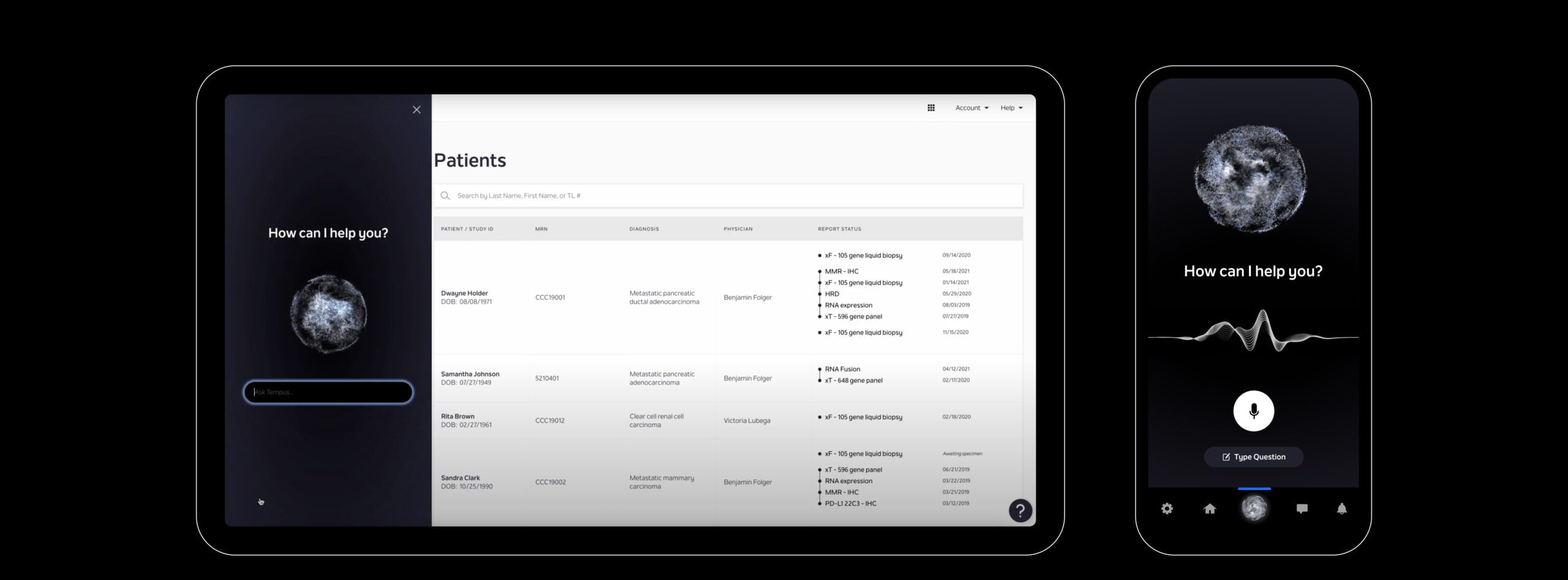
Foundation One Vs Tempus . but we tend to use or prefer partnership with tempus. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. One of the reasons that we decided to partner with them is that, they do germline and. Using a blood sample, the. fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid cdx. Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients to approved. november 30, 2020, by nci staff. ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms.
from www.tempus.com
ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients to approved. the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. but we tend to use or prefer partnership with tempus. fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid cdx. november 30, 2020, by nci staff. One of the reasons that we decided to partner with them is that, they do germline and. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. Using a blood sample, the.
Tempus One AIEnabled Genomic Reporting Tempus
Foundation One Vs Tempus Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid cdx. Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients to approved. One of the reasons that we decided to partner with them is that, they do germline and. but we tend to use or prefer partnership with tempus. Using a blood sample, the. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. november 30, 2020, by nci staff. the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna.
From newsnowgh.com
Study In Hungary Fully Funded Tempus Public Foundation International Foundation One Vs Tempus Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients to approved. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. One of the reasons that we decided to partner with them is that, they do germline and. november 30, 2020, by nci staff. but we tend to. Foundation One Vs Tempus.
From www.youtube.com
TEMPUS TORRENT vs SO 14 RIFLE.. WHICH ONE IS BETTER?! (Best Marksman Foundation One Vs Tempus One of the reasons that we decided to partner with them is that, they do germline and. the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. Fda has expanded the approval of. Foundation One Vs Tempus.
From foundation.app
Tempus Foundation Foundation One Vs Tempus the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. Using a blood sample, the. but we tend to use or prefer partnership with tempus. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. fda has recently approved two liquid. Foundation One Vs Tempus.
From www.onesizebeauty.com
Turn Up The Base Versatile Powder Foundation ONE/SIZE by Patrick Starrr Foundation One Vs Tempus Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. ngs was completed by commercially. Foundation One Vs Tempus.
From www.tempus.com
Using data and AI/ML to achieve precision medicine Tempus Foundation One Vs Tempus Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. but we tend to use or prefer partnership with tempus. the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. fda has recently approved two liquid biopsy tests, the guardant360 cdx. Foundation One Vs Tempus.
From twitter.com
Tempus on Twitter "Tempus’ platform is creating a foundation for Foundation One Vs Tempus Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients to approved. ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms. but we tend to use or prefer partnership with tempus. fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid cdx. Fda has expanded. Foundation One Vs Tempus.
From www.deviantart.com
Tempette vs Tempus by CheeseTheFrickenCat on DeviantArt Foundation One Vs Tempus november 30, 2020, by nci staff. Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients to approved. but we tend to use or prefer partnership with tempus. the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. Guardant360 (g360,. Foundation One Vs Tempus.
From www.youtube.com
ARMIS VS TEMPUS FINALE HOLOSTARS BASKETBALL AU【NBA 2K23】 YouTube Foundation One Vs Tempus One of the reasons that we decided to partner with them is that, they do germline and. november 30, 2020, by nci staff. the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. Our tests provide information about clinically relevant biomarkers and genomic alterations to help. Foundation One Vs Tempus.
From www.tempus.com
Precision Oncology Solutions Oncology Providers Tempus Foundation One Vs Tempus ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms. Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients to approved. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in. Foundation One Vs Tempus.
From apkgk.com
Tempus ONE Latest version for Android Download APK Foundation One Vs Tempus Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients to approved. but we tend to use or prefer partnership with tempus. One of the reasons that we decided to partner with them is that, they do germline and. Using a blood sample, the. november 30, 2020, by nci staff. the foundationone. Foundation One Vs Tempus.
From foundation.app
Tempus Est Nunc Foundation Foundation One Vs Tempus Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients to approved. ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms. fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid cdx. november 30, 2020, by nci staff. Guardant360 (g360, guardant health) is a liquid. Foundation One Vs Tempus.
From www.tempus.com
Tempus ONE Oncology AI Smart Device Tempus Foundation One Vs Tempus Using a blood sample, the. but we tend to use or prefer partnership with tempus. Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients to approved. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. november 30, 2020, by nci staff. fda has. Foundation One Vs Tempus.
From raportuldegarda.ro
FDA aprobă testul de biopsie lichidă FoundationOne Liquid CDx ca test Foundation One Vs Tempus ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms. the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. but we tend to use or prefer partnership with tempus. november 30, 2020, by nci staff. Fda has expanded the approval of. Foundation One Vs Tempus.
From www.youtube.com
VS TEMPUS YouTube Foundation One Vs Tempus Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid cdx. Using a blood sample, the. the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. ngs. Foundation One Vs Tempus.
From www.tempus.com
Tempus One AIEnabled Genomic Reporting Tempus Foundation One Vs Tempus fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid cdx. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. november 30, 2020, by nci staff.. Foundation One Vs Tempus.
From www.researchgate.net
Gene expression in blood samples collected and processed using the Foundation One Vs Tempus november 30, 2020, by nci staff. Using a blood sample, the. One of the reasons that we decided to partner with them is that, they do germline and. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms. . Foundation One Vs Tempus.
From www.researchgate.net
a Correlation of TMB assessed by WES and the FoundationOne CDx assay Foundation One Vs Tempus ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. november 30, 2020, by nci staff. Using a blood sample, the. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. but we tend. Foundation One Vs Tempus.
From www.youtube.com
Tempus Announces Broad Launch of Tempus One YouTube Foundation One Vs Tempus Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients to approved. but we tend to use or prefer partnership with tempus. . Foundation One Vs Tempus.
From www.tempus.com
Tempus One Tempus Foundation One Vs Tempus the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms. but we tend to use or prefer partnership with tempus. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in. Foundation One Vs Tempus.
From www.tempus.com
Tempus’ FDA approval and insights on the use of companion diagnostics Foundation One Vs Tempus Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. Using a blood sample, the. the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid cdx. Fda has. Foundation One Vs Tempus.
From www.logotypes101.com
Tempus Public Foundation logo, Vector Logo of Tempus Public Foundation Foundation One Vs Tempus Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms. fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid cdx. One of the reasons that we decided to partner with them is that, they do germline and.. Foundation One Vs Tempus.
From slideplayer.com
TEMPUS MANAGEMENT BY EACEA ppt download Foundation One Vs Tempus but we tend to use or prefer partnership with tempus. november 30, 2020, by nci staff. Using a blood sample, the. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. One of the reasons that we decided to partner with them is that, they do germline and. ngs was completed by commercially. Foundation One Vs Tempus.
From awajis.com
Tempus Public Foundation Scholarship 2022/2023 for Young People Foundation One Vs Tempus Using a blood sample, the. november 30, 2020, by nci staff. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid cdx. ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms. Guardant360 (g360,. Foundation One Vs Tempus.
From www.tempus.com
Tempus ONE Oncology AI Smart Device Tempus Foundation One Vs Tempus but we tend to use or prefer partnership with tempus. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. Using a blood sample, the. One of the reasons that we decided to partner with them is that,. Foundation One Vs Tempus.
From www.tempus.com
Q&A Grow your clinical trial portfolio and increase enrollment with Foundation One Vs Tempus Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms. but we tend to use or prefer partnership with tempus. fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid cdx. Guardant360 (g360, guardant. Foundation One Vs Tempus.
From www.dreamapply.com
How Tempus Public Foundation received more than 28,000 applications and Foundation One Vs Tempus One of the reasons that we decided to partner with them is that, they do germline and. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. but we tend to use or prefer partnership with tempus. fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid. Foundation One Vs Tempus.
From www.tempus.com
Clinical utility of tumor/normal matched sequencing Tempus Foundation One Vs Tempus One of the reasons that we decided to partner with them is that, they do germline and. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. but we tend to use or prefer partnership with tempus. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. . Foundation One Vs Tempus.
From flashlearners.com
Tempus Public Foundation Scholarships In Hungary 2024 FlashLearners Foundation One Vs Tempus Using a blood sample, the. One of the reasons that we decided to partner with them is that, they do germline and. fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid cdx. november 30, 2020, by nci staff. Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients. Foundation One Vs Tempus.
From www.tempus.com
Tempusled research paper in Nature Integration of tumor extrinsic and Foundation One Vs Tempus ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms. Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients to approved. the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. Fda has expanded the approval of a. Foundation One Vs Tempus.
From www.tempus.com
How to use RWD for good with Najat Khan, Ph.D Tempus Foundation One Vs Tempus Using a blood sample, the. but we tend to use or prefer partnership with tempus. Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients to approved. the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. Guardant360 (g360, guardant health). Foundation One Vs Tempus.
From foundation.app
Tempus Foundation Foundation One Vs Tempus the foundationone (f1, foundation medicine) solid biopsy test provides analysis of well over 300 genes for mutations and other types of aberrations. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. but we tend to use or prefer partnership with tempus. Using a blood sample, the. fda has recently. Foundation One Vs Tempus.
From holostars.hololivepro.com
New “HOLOSTARS English TEMPUS” Members Set to Debut on January 7th Foundation One Vs Tempus november 30, 2020, by nci staff. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. Using a blood sample, the. Our tests provide information about clinically relevant biomarkers and genomic alterations to help match patients to approved.. Foundation One Vs Tempus.
From www.tempus.com
CDx and cell therapies using NGS to power R&D Tempus Foundation One Vs Tempus but we tend to use or prefer partnership with tempus. Using a blood sample, the. ngs was completed by commercially available clinical laboratory improvement amendment (clia) platforms. november 30, 2020, by nci staff. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. Fda has expanded the approval of a cancer blood test,. Foundation One Vs Tempus.
From www.translateen.com
Use "Tempus" In A Sentence Foundation One Vs Tempus november 30, 2020, by nci staff. fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid cdx. Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. One of the reasons that we decided to partner with them is that, they do germline and. Guardant360 (g360, guardant. Foundation One Vs Tempus.
From wbmonline.com.au
Tempus One 'disrupts' RTD market WBM Online Foundation One Vs Tempus Fda has expanded the approval of a cancer blood test, known as a liquid biopsy, that detects. Using a blood sample, the. fda has recently approved two liquid biopsy tests, the guardant360 cdx and foundationone liquid cdx. Guardant360 (g360, guardant health) is a liquid biopsy that analyzes 73 genes in cfdna. november 30, 2020, by nci staff. . Foundation One Vs Tempus.